A role of actin filament in synaptic transmission and long-term potentiation
- PMID: 10341235
- PMCID: PMC6782630
- DOI: 10.1523/JNEUROSCI.19-11-04314.1999
A role of actin filament in synaptic transmission and long-term potentiation
Abstract
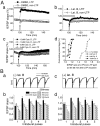
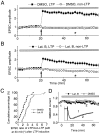
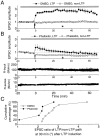
The role of actin filaments in synaptic function has been studied in the CA1 region of the rat hippocampal slice. Bath application (2 hr) of the actin polymerization inhibitor latrunculin B did not substantially affect the shape of dendrites or spines. However, this and other drugs that affect actin did affect synaptic function. Bath-applied latrunculin B reduced the synaptic response. Several lines of evidence indicate that a component of this effect is presynaptic. To specifically test for a postsynaptic role for actin, latrunculin B or phalloidin, an actin filament stabilizer, was perfused into the postsynaptic neuron. The magnitude of long-term potentiation (LTP) was decreased at times when baseline transmission was not yet affected. Longer applications produced a decrease in baseline AMPA receptor (AMPAR)-mediated transmission. The magnitude of the NMDA receptor-mediated transmission was unaffected, indicating a specific effect on the AMPAR. These results suggest that postsynaptic actin filaments are involved in a dynamic process required to maintain AMPAR-mediated transmission and to enhance it during LTP.
Figures






Similar articles
-
Actin polymerization regulates the synthesis of PKMzeta in LTP.Neuropharmacology. 2007 Jan;52(1):41-5. doi: 10.1016/j.neuropharm.2006.07.002. Epub 2006 Aug 17. Neuropharmacology. 2007. PMID: 16914172
-
Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses.Hippocampus. 2010 May;20(5):646-58. doi: 10.1002/hipo.20654. Hippocampus. 2010. PMID: 19489005
-
Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors.J Neurosci. 1998 Apr 1;18(7):2423-36. doi: 10.1523/JNEUROSCI.18-07-02423.1998. J Neurosci. 1998. PMID: 9502803 Free PMC article.
-
The role of actin in the regulation of dendritic spine morphology and bidirectional synaptic plasticity.Neuroreport. 2004 Apr 9;15(5):829-32. doi: 10.1097/00001756-200404090-00018. Neuroreport. 2004. PMID: 15073524
-
Dual pools of actin at presynaptic terminals.J Neurophysiol. 2012 Jun;107(12):3479-92. doi: 10.1152/jn.00789.2011. Epub 2012 Mar 28. J Neurophysiol. 2012. PMID: 22457456 Free PMC article.
Cited by
-
A delayed response enhancement during hippocampal presynaptic plasticity in mice.J Physiol. 2007 Aug 15;583(Pt 1):129-43. doi: 10.1113/jphysiol.2007.131300. Epub 2007 Jun 14. J Physiol. 2007. PMID: 17569738 Free PMC article.
-
Developmental regulation of spine motility in the mammalian central nervous system.Proc Natl Acad Sci U S A. 1999 Nov 9;96(23):13438-43. doi: 10.1073/pnas.96.23.13438. Proc Natl Acad Sci U S A. 1999. PMID: 10557339 Free PMC article.
-
Dendritic spine viscoelasticity and soft-glassy nature: balancing dynamic remodeling with structural stability.Biophys J. 2007 Feb 15;92(4):1419-30. doi: 10.1529/biophysj.106.092361. Epub 2006 Nov 17. Biophys J. 2007. PMID: 17114228 Free PMC article.
-
Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses.J Neurosci. 2006 Aug 30;26(35):8881-91. doi: 10.1523/JNEUROSCI.1302-06.2006. J Neurosci. 2006. PMID: 16943543 Free PMC article.
-
GluR1 links structural and functional plasticity at excitatory synapses.J Neurosci. 2007 Dec 12;27(50):13706-18. doi: 10.1523/JNEUROSCI.3503-07.2007. J Neurosci. 2007. PMID: 18077682 Free PMC article.
References
-
- Bernstein BW, Bamburg JR. Cycling of actin assembly in synaptosomes and neurotransmitter release. Neuron. 1989;3:257–265. - PubMed
-
- Bortolotto ZA, Collingridge GL. Involvement of calcium/calmodulin-dependent protein kinases in the setting of a molecular switch involved in hippocampal LTP. Neuropharmacology. 1998;37:535–544. - PubMed
-
- Calakos N, Scheller RH. Synaptic vesicle biogenesis, docking, and fusion: a molecular description. Physiol Rev. 1996;76:1–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
