Calcium-induced calcium release contributes to action potential-evoked calcium transients in hippocampal CA1 pyramidal neurons
- PMID: 10341236
- PMCID: PMC6782593
- DOI: 10.1523/JNEUROSCI.19-11-04325.1999
Calcium-induced calcium release contributes to action potential-evoked calcium transients in hippocampal CA1 pyramidal neurons
Abstract
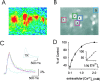
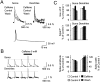
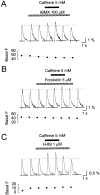
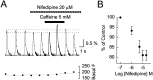
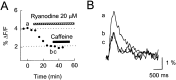
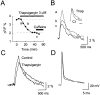
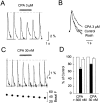
Calcium-induced calcium release (CICR) is a mechanism by which local elevations of intracellular calcium (Ca2+) are amplified by Ca2+ release from ryanodine-sensitive Ca2+ stores. CICR is known to be coupled to Ca2+ entry in skeletal muscle, cardiac muscle, and peripheral neurons, but no evidence suggests that such coupling occurs in central neurons during the firing of action potentials. Using fast Ca2+ imaging in CA1 neurons from hippocampal slices, we found evidence for CICR during action potential-evoked Ca2+ transients. A low concentration of caffeine enhanced Ca2+ transient amplitude, whereas a higher concentration reduced it. Simultaneous Ca2+ imaging and whole-cell recordings showed that membrane potential, action potential amplitude, and waveform were unchanged during caffeine application. The enhancement of Ca2+ transients by caffeine was not affected by the L-type channel blocker nifedipine, the phosphodiesterase inhibitor IBMX, the adenylyl cyclase activator forskolin, or the PKA antagonist H-89. However, thapsigargin or ryanodine, which both empty intracellular Ca2+ stores, occluded this effect. In addition, thapsigargin, ryanodine, and cyclopiazonic acid reduced action potential-evoked Ca2+ transients in the absence of caffeine. These results suggest that Ca2+ release from ryanodine-sensitive stores contributes to Ca2+ signals triggered by action potentials in CA1 neurons.
Figures



Similar articles
-
Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones.J Physiol. 1997 Jul 1;502 ( Pt 1)(Pt 1):13-30. doi: 10.1111/j.1469-7793.1997.013bl.x. J Physiol. 1997. PMID: 9234194 Free PMC article.
-
Characterization of action potential-triggered [Ca2+]i transients in single smooth muscle cells of guinea-pig ileum.Br J Pharmacol. 1997 Oct;122(3):477-86. doi: 10.1038/sj.bjp.0701407. Br J Pharmacol. 1997. PMID: 9351504 Free PMC article.
-
Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons.J Neurosci. 2002 May 1;22(9):3454-62. doi: 10.1523/JNEUROSCI.22-09-03454.2002. J Neurosci. 2002. PMID: 11978822 Free PMC article.
-
Ryanodine receptor-mediated intracellular calcium release in rat cerebellar Purkinje neurones.J Physiol. 1995 Aug 15;487(1):1-16. doi: 10.1113/jphysiol.1995.sp020857. J Physiol. 1995. PMID: 7473240 Free PMC article.
-
Calcium-induced calcium release in skeletal muscle.Physiol Rev. 2009 Oct;89(4):1153-76. doi: 10.1152/physrev.00040.2008. Physiol Rev. 2009. PMID: 19789379 Review.
Cited by
-
The potential of caffeine for functional modification from cortical synapses to neuron networks in the brain.Curr Neuropharmacol. 2005 Oct;3(4):309-16. doi: 10.2174/157015905774322543. Curr Neuropharmacol. 2005. PMID: 18369398 Free PMC article.
-
Assay of Calcium Transients and Synapses in Rat Hippocampal Neurons by Kinetic Image Cytometry and High-Content Analysis: An In Vitro Model System for Postchemotherapy Cognitive Impairment.Assay Drug Dev Technol. 2017 Jul;15(5):220-236. doi: 10.1089/adt.2017.797. Assay Drug Dev Technol. 2017. PMID: 28723268 Free PMC article.
-
Calcium-induced calcium release and type 3 ryanodine receptors modulate the slow afterhyperpolarising current, sIAHP, and its potentiation in hippocampal pyramidal neurons.PLoS One. 2020 Jun 19;15(6):e0230465. doi: 10.1371/journal.pone.0230465. eCollection 2020. PLoS One. 2020. PMID: 32559219 Free PMC article.
-
The effect of caffeine supplementation on muscular strength and endurance: A meta-analysis of meta-analyses.Heliyon. 2024 Jul 25;10(15):e35025. doi: 10.1016/j.heliyon.2024.e35025. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170391 Free PMC article.
-
Aging-Related Alterations to Persistent Firing in the Lateral Entorhinal Cortex Contribute to Deficits in Temporal Associative Memory.Front Aging Neurosci. 2022 Mar 11;14:838513. doi: 10.3389/fnagi.2022.838513. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35360205 Free PMC article. Review.
References
-
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. - PubMed
-
- Beavo JA, Reifsnyder DH. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990;11:150–155. - PubMed
-
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
-
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium–response curves of Ins1,4,5P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
