Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function
- PMID: 10341240
- PMCID: PMC6782629
- DOI: 10.1523/JNEUROSCI.19-11-04370.1999
Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function
Abstract
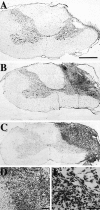
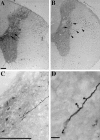
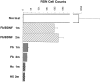
Adult mammalian CNS neurons do not normally regenerate their severed axons. This failure has been attributed to scar tissue and inhibitory molecules at the injury site that block the regenerating axons, a lack of trophic support for the axotomized neurons, and intrinsic neuronal changes that follow axotomy, including cell atrophy and death. We studied whether transplants of fibroblasts genetically engineered to produce brain-derived neurotrophic factor (BDNF) would promote rubrospinal tract (RST) regeneration in adult rats. Primary fibroblasts were modified by retroviral-mediated transfer of a DNA construct encoding the human BDNF gene, an internal ribosomal entry site, and a fusion gene of lacZ and neomycin resistance genes. The modified fibroblasts produce biologically active BDNF in vitro. These cells were grafted into a partial cervical hemisection cavity that completely interrupted one RST. One and two months after lesion and transplantation, RST regeneration was demonstrated with retrograde and anterograde tracing techniques. Retrograde tracing with fluorogold showed that approximately 7% of RST neurons regenerated axons at least three to four segments caudal to the transplants. Anterograde tracing with biotinylated dextran amine revealed that the RST axons regenerated through and around the transplants, grew for long distances within white matter caudal to the transplant, and terminated in spinal cord gray matter regions that are the normal targets of RST axons. Transplants of unmodified primary fibroblasts or Gelfoam alone did not elicit regeneration. Behavioral tests demonstrated that recipients of BDNF-producing fibroblasts showed significant recovery of forelimb usage, which was abolished by a second lesion that transected the regenerated axons.
Figures










Similar articles
-
Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury.Exp Neurol. 2002 Sep;177(1):265-75. doi: 10.1006/exnr.2002.7980. Exp Neurol. 2002. PMID: 12429228
-
Human adult olfactory neural progenitors rescue axotomized rodent rubrospinal neurons and promote functional recovery.Exp Neurol. 2005 Jul;194(1):12-30. doi: 10.1016/j.expneurol.2005.01.021. Exp Neurol. 2005. PMID: 15899240
-
Grafts of BDNF-producing fibroblasts rescue axotomized rubrospinal neurons and prevent their atrophy.Exp Neurol. 2002 Dec;178(2):150-64. doi: 10.1006/exnr.2002.7977. Exp Neurol. 2002. PMID: 12504875
-
Transplantation of genetically modified cells contributes to repair and recovery from spinal injury.Brain Res Brain Res Rev. 2002 Oct;40(1-3):292-300. doi: 10.1016/s0165-0173(02)00211-4. Brain Res Brain Res Rev. 2002. PMID: 12589927 Review.
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
Cited by
-
Expression of heat shock protein (HSP)-25 and HSP-32 in the rat spinal cord reconstructed with Neurogel.Neurochem Res. 2005 Jun-Jul;30(6-7):721-35. doi: 10.1007/s11064-005-6866-8. Neurochem Res. 2005. PMID: 16187209
-
Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity.Philos Trans R Soc Lond B Biol Sci. 2006 Sep 29;361(1473):1611-34. doi: 10.1098/rstb.2006.1890. Philos Trans R Soc Lond B Biol Sci. 2006. PMID: 16939978 Free PMC article. Review.
-
Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A.J Neurosci. 2001 May 15;21(10):3665-73. doi: 10.1523/JNEUROSCI.21-10-03665.2001. J Neurosci. 2001. PMID: 11331396 Free PMC article.
-
Targeting a dominant negative rho kinase to neurons promotes axonal outgrowth and partial functional recovery after rat rubrospinal tract lesion.Mol Ther. 2009 Dec;17(12):2020-30. doi: 10.1038/mt.2009.168. Epub 2009 Jul 21. Mol Ther. 2009. PMID: 19623163 Free PMC article.
-
Effect of cervical dorsolateral funiculotomy on reach-to-grasp function in the rat.J Neurotrauma. 2008 Aug;25(8):1039-47. doi: 10.1089/neu.2007.0419. J Neurotrauma. 2008. PMID: 18721108 Free PMC article.
References
-
- Bernstein-Goral H, Bregman BS. Spinal cord transplants support the regeneration of axotomized neurons after spinal cord lesions at birth: a quantitative double-labeling study. Exp Neurol. 1993;123:118–132. - PubMed
-
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. - PubMed
-
- Bregman BS, McAtee M, Dai HN, Kuhn PL. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol. 1997;148:475–494. - PubMed
-
- Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. - PubMed
-
- Brown LT. Rubrospinal projections in the rat. J Comp Neurol. 1974;154:169–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
