Anatomical correlates of functional plasticity in mouse visual cortex
- PMID: 10341241
- PMCID: PMC2452998
- DOI: 10.1523/JNEUROSCI.19-11-04388.1999
Anatomical correlates of functional plasticity in mouse visual cortex
Abstract
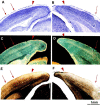
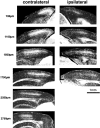
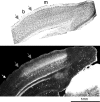
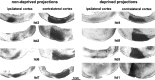
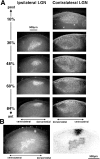
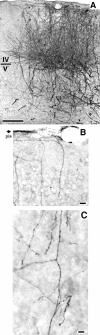
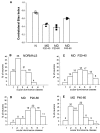
Much of what is known about activity-dependent plasticity comes from studies of the primary visual cortex and its inputs in higher mammals, but the molecular bases remain largely unknown. Similar functional plasticity takes place during a critical period in the visual cortex of the mouse, an animal in which genetic experiments can readily be performed to investigate the underlying molecular and cellular events. The experiments of this paper were directed toward understanding whether anatomical changes accompany functional plasticity in the developing visual cortex of the mouse, as they do in higher mammals. In normal mice, transneuronal label after an eye injection clearly delineated the monocular and binocular zones of area 17. Intrinsic signal optical imaging also showed monocular and binocular zones of area 17 but revealed no finer organization of ocular dominance or orientation selectivity. In normal animals, single geniculocortical afferents serving the contralateral eye showed great heterogeneity and no clustering consistent with the presence of ocular dominance patches. Growth and elaboration of terminal arbor continues beyond postnatal day 40 (P40), after the peak of the critical period. After prolonged monocular deprivation (MD) from P20 to P60, transneuronal labeling showed that the projection serving the ipsilateral eye was severely affected, whereas the effect on the contralateral eye's pathway was inconsistent. Optical imaging also showed profound effects of deprivation, particularly in the ipsilateral pathway, and microelectrode studies confirmed continued functional plasticity past P40. Reconstruction of single afferents showed that MD from P20 to P40 promoted the growth of the open eye's geniculocortical connections without causing the closed eye's contralateral projection to shrink, whereas MD from P20 to P60 caused an arrest of growth of deprived arbors. Our findings reveal numerous similarities between mouse and higher mammals in development and plasticity, along with some differences. We discuss the factors that may be responsible for these differences.
Figures






References
-
- Antonini A, Stryker MP. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion the cat. J Comp Neurol. 1996;369:64–82. - PubMed
-
- Blakemore C, Vital-Durand F, Garey LJ. Recovery from monocular deprivation in the monkey. I. Reversal of physiological effects in the visual cortex. Proc R Soc Lond B Biol Sci. 1981;213:399–423. - PubMed
-
- Bonhoeffer T, Grinvald A. Optical imaging of the functional architecture in cat visual cortex: the layout of direction and orientation domains. Adv Exp Med Biol. 1993;333:57–69. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
