Collapsin-1/semaphorin-III/D is regulated developmentally in Purkinje cells and collapses pontocerebellar mossy fiber neuronal growth cones
- PMID: 10341245
- PMCID: PMC6782633
- DOI: 10.1523/JNEUROSCI.19-11-04437.1999
Collapsin-1/semaphorin-III/D is regulated developmentally in Purkinje cells and collapses pontocerebellar mossy fiber neuronal growth cones
Abstract
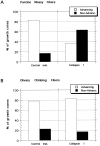
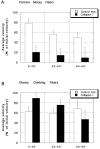
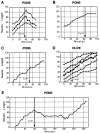
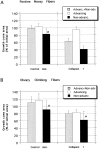
Most axons in the CNS innervate specific subregions or layers of their target regions and form contacts with specific types of target neurons, but the molecular basis of this process is not well understood. To determine whether collapsin-1/semaphorin-III/D, a molecule known to repel specific axons, might guide afferent axons within their cerebellar targets, we characterized its expression by in situ hybridization and observed its effects on mossy and climbing fiber extension and growth cone size in vitro. In newborn mice sema-D is expressed by cerebellar Purkinje cells in parasagittal bands located medially and in some cells of the cerebellar nuclei. Later, sema-D expression in Purkinje cells broadens such that banded expression is no longer prominent, and expression is detected in progressively more lateral regions. By postnatal day 16, expression is observed throughout the cerebellar mediolateral axis. Collapsin-1 protein, the chick ortholog of sema-D, did not inhibit the extension of neurites from explants of inferior olivary nuclei, the source of climbing fibers that innervate Purkinje cells. In contrast, when it was applied to axons extending from basilar pontine explants, a source of mossy fiber afferents of granule cells, collapsin-1 caused most pontine growth cones to collapse, as evidenced by a reduction in growth cone size of up to 59%. Moreover, 63% of pontine growth cones arrested their extension or retracted. Its effects on mossy fiber extension and its distribution suggest that sema-D prevents mossy fibers from innervating inappropriate cerebellar target regions and cell types.
Figures


Similar articles
-
Many major CNS axon projections develop normally in the absence of semaphorin III.Mol Cell Neurosci. 1998 Jul;11(4):173-82. doi: 10.1006/mcne.1998.0687. Mol Cell Neurosci. 1998. PMID: 9675049
-
Cerebellar target neurons provide a stop signal for afferent neurite extension in vitro.J Neurosci. 1992 Feb;12(2):619-34. doi: 10.1523/JNEUROSCI.12-02-00619.1992. J Neurosci. 1992. PMID: 1740694 Free PMC article.
-
Zebrafish semaphorin Z1a collapses specific growth cones and alters their pathway in vivo.Development. 1998 Apr;125(7):1275-83. doi: 10.1242/dev.125.7.1275. Development. 1998. PMID: 9477326
-
Climbing fibers mediate vestibular modulation of both "complex" and "simple spikes" in Purkinje cells.Cerebellum. 2015 Oct;14(5):597-612. doi: 10.1007/s12311-015-0725-1. Cerebellum. 2015. PMID: 26424151 Review.
-
Climbing fiber development: do neurotrophins have a part to play?Cerebellum. 2002 Dec;1(4):265-75. doi: 10.1080/147342202320883579. Cerebellum. 2002. PMID: 12879965 Review.
Cited by
-
Expression patterns of semaphorin7A and plexinC1 during rat neural development suggest roles in axon guidance and neuronal migration.BMC Dev Biol. 2007 Aug 29;7:98. doi: 10.1186/1471-213X-7-98. BMC Dev Biol. 2007. PMID: 17727705 Free PMC article.
-
Cell death as a regulator of cerebellar histogenesis and compartmentation.Cerebellum. 2011 Sep;10(3):373-92. doi: 10.1007/s12311-010-0222-5. Cerebellum. 2011. PMID: 20941559 Review.
-
Oligodendrocytes as regulators of neuronal networks during early postnatal development.PLoS One. 2011 May 12;6(5):e19849. doi: 10.1371/journal.pone.0019849. PLoS One. 2011. PMID: 21589880 Free PMC article.
-
The pathogenesis of spinocerebellar ataxia.Cerebellum. 2005;4(1):62-73. doi: 10.1080/14734220510007950. Cerebellum. 2005. PMID: 15895563 Review.
-
Inhibition of Sema-3A Promotes Cell Migration, Axonal Growth, and Retinal Ganglion Cell Survival.Transl Vis Sci Technol. 2021 Aug 12;10(10):16. doi: 10.1167/tvst.10.10.16. Transl Vis Sci Technol. 2021. PMID: 34817617 Free PMC article.
References
-
- Adams RH, Betz H, Püschel AW. A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mech Dev. 1996;57:33–45. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. III. Maturation of the components of the granule layer. J Comp Neurol. 1972;145:465–514. - PubMed
-
- Altman J, Bayer SA. Development of the precerebellar nuclei in the rat. IV. The anterior precerebellar extramural migratory stream and the nucleus reticularis tegmenti pontis and the basal pontine gray. J Comp Neurol. 1987;257:529–552. - PubMed
-
- Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure and functions. CRC; New York: 1996.
-
- Azizi SA, Mihailoff GA, Burne RA, Woodward DJ. The pontocerebellar system in the rat: an HRP study. I. Posterior vermis. J Comp Neurol. 1981;197:543–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
