Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration
- PMID: 10341246
- PMCID: PMC6782619
- DOI: 10.1523/JNEUROSCI.19-11-04449.1999
Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration
Abstract
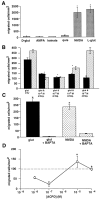
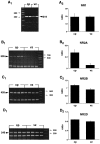
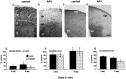
During cortical development, embryonic neurons migrate from germinal zones near the ventricle into the cortical plate, where they organize into layers. Mechanisms that direct neuronal migration may include molecules that act as chemoattractants. In rats, GABA, which localizes near the target destination for migrating cortical neurons, stimulates embryonic neuronal migration in vitro. In mice, glutamate is highly localized near the target destinations for migrating cortical neurons. Glutamate-induced migration of murine embryonic cortical cells was evaluated in cell dissociates and cortical slice cultures. In dissociates, the chemotropic effects of glutamate were 10-fold greater than the effects of GABA, demonstrating that for murine cortical cells, glutamate is a more potent chemoattractant than GABA. Thus, cortical chemoattractants appear to differ between species. Micromolar glutamate stimulated neuronal chemotaxis that was mimicked by microM NMDA but not by other ionotropic glutamate receptor agonists (AMPA, kainate, quisqualate). Responding cells were primarily derived from immature cortical regions [ventricular zone (vz)/subventricular zone (svz)]. Bromodeoxyuridine (BrdU) pulse labeling of cortical slices cultured in NMDA antagonists (microM MK801 or APV) revealed that antagonist exposure blocked the migration of BrdU-positive cells from the vz/svz into the cortical plate. PCR confirmed the presence of NMDA receptor expression in vz/svz cells, whereas electrophysiology and Ca2+ imaging demonstrated that vz/svz cells exhibited physiological responses to NMDA. These studies indicate that, in mice, glutamate may serve as a chemoattractant for neurons in the developing cortex, signaling cells to migrate into the cortical plate via NMDA receptor activation.
Figures





References
-
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
-
- Armstrong RC, Harvath L, Dubois-Dalcq M. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–407. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
