Supporting cells contribute to control of hearing sensitivity
- PMID: 10341250
- PMCID: PMC6782614
- DOI: 10.1523/JNEUROSCI.19-11-04498.1999
Supporting cells contribute to control of hearing sensitivity
Abstract
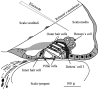
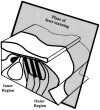
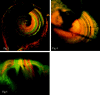
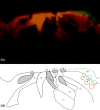
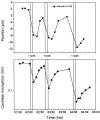
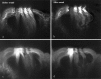
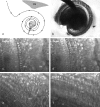
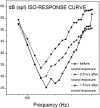
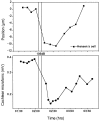
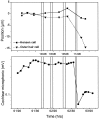
The mammalian hearing organ, the organ of Corti, was studied in an in vitro preparation of the guinea pig temporal bone. As in vivo, the hearing organ responded with an electrical potential, the cochlear microphonic potential, when stimulated with a test tone. After exposure to intense sound, the response to the test tone was reduced. The electrical response either recovered within 10-20 min or remained permanently reduced, thus corresponding to a temporary or sustained loss of sensitivity. Using laser scanning confocal microscopy, stimulus-induced changes of the cellular structure of the hearing organ were simultaneously studied. The cells in the organ were labeled with two fluorescent probes, a membrane dye and a cytoplasm dye, showing enzymatic activity in living cells. Confocal microscopy images were collected and compared before and after intense sound exposure. The results were as follows. (1) The organ of Corti could be divided into two different structural entities in terms of their susceptibility to damage: an inner, structurally stable region comprised of the inner hair cell with its supporting cells and the inner and outer pillar cells; and an outer region that exhibited dynamic structural changes and consisted of the outer hair cells and the third Deiters' cell with its attached Hensen's cells. (2) Exposure to intense sound caused the Deiters' cells and Hensen's cells to move in toward the center of the cochlear turn. (3) This event coincided with a reduced sensitivity to the test tone (i.e., reduced cochlear microphonic potential). (4) The displacement and sensitivity loss could be reversible. It is concluded that these observations have relevance for understanding the mechanisms behind hearing loss after noise exposure and that the supporting cells take an active part in protection against trauma during high-intensity sound exposure.
Figures
References
-
- Borg E, Canlon B, Engström B. Noise induced hearing loss: literature review and experiments in rabbits. Scand Audiol. 1995;24:1–147. - PubMed
-
- Brundin L, Flock B, Flock Å. Sound induced displacement responses of the guinea pig hearing organ and its relation to the cochlear potentials. Hear Res. 1992;58:175–184. - PubMed
-
- Canlon B. Acoustic overstimulation alters the morphology of the membrana tectoria. Hear Res. 1987;30:127–134. - PubMed
-
- Cody AR, Russell IJ. OHCs in the mammalian cochlea and noise-induced hearing loss. Nature. 1985;315:662–665. - PubMed
-
- Dulon D, Blanchet C, Laffon E. Photo-released intracellular Ca2+ evokes reversible mechanical responses in supporting cells of the guinea pig organ of Corti. Biochem Biophys Res Commun. 1994;3:1263–1269. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
