Odor coding in a model olfactory organ: the Drosophila maxillary palp
- PMID: 10341252
- PMCID: PMC6782632
- DOI: 10.1523/JNEUROSCI.19-11-04520.1999
Odor coding in a model olfactory organ: the Drosophila maxillary palp
Abstract
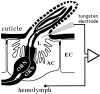
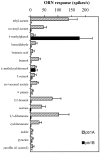
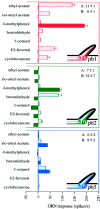
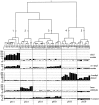
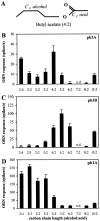
Odor coding relies on the activity of different classes of receptor neurons, each with distinct response characteristics. We have examined odor coding in a model olfactory organ, the maxillary palp of Drosophila. This organ contains only 120 olfactory receptor neurons, compartmentalized in sensory hairs called sensilla, and provides an opportunity to characterize all neurons in an entire olfactory organ. Extensive extracellular recordings from single sensilla reveal that the neurons fall into six functional classes. Each of the 60 sensilla houses two neurons, which observe a pairing rule: each sensillum combines neurons of two particular classes, thereby yielding three sensillum types. The sensillum types are intermingled on the surface of the palp, but their distribution is not random. The neurons exhibit diverse response characteristics, providing the basis for an olfactory code. A particular odor can excite one neuron and inhibit another, and a particular neuron can be excited by one odor and inhibited by another. Some excitatory responses continue beyond the end of odor delivery, but responses to most odors terminate abruptly after the end of odor delivery, with some followed by a period of poststimulus quiescence. The specificity of odor response is examined in detail for the neurons of one sensillum, which were found to differ in their relative responses to a homologous series of esters. Adaptation and cross-adaptation are documented, and cross-adaptation experiments demonstrate that the two neurons within one type of sensillum can function independently. The analysis of all neuronal types in this model olfactory organ is discussed in terms of its functional organization and the mechanisms by which it encodes olfactory information.
Figures






References
-
- Ache BW, Zhainazarov A. Dual second-messenger pathways in olfactory transduction. Curr Opin Neurobiol. 1995;5:461–466. - PubMed
-
- Almaas TJ, Christensen TA, Mustaparta H. Chemical communication in heliothine moths. I. Antennal receptor neurons encode several features of intra- and interspecific odorants in the male corn earworm moth Helicoverpa zea. J Comp Physiol [A] 1991;169:249–258.
-
- Altner H, Prillinger L. Ultrastructure of invertebrate chemo-, thermo- and hygroreceptors and its functional significance. Int Rev Cytol. 1980;67:69–139.
-
- Anderberg MR. Cluster analysis for applications. Academic; New York: 1973.
-
- Anderson P, Hansson BS, Löfqvist J. Plant-odour-specific receptor neurones on the antennae of female and male Spodoptera littoralis. Physiol Entomol. 1995;20:189–198.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
