Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states
- PMID: 10341257
- PMCID: PMC6782626
- DOI: 10.1523/JNEUROSCI.19-11-04595.1999
Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states
Abstract
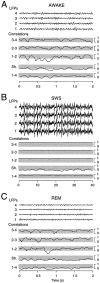
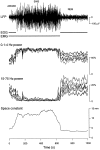
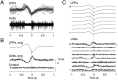
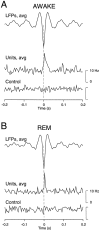
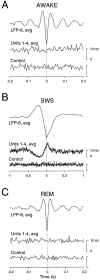
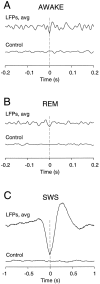
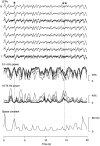
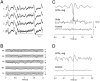
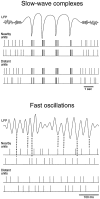
The electroencephalogram displays various oscillation patterns during wake and sleep states, but their spatiotemporal distribution is not completely known. Local field potentials (LFPs) and multiunits were recorded simultaneously in the cerebral cortex (areas 5-7) of naturally sleeping and awake cats. Slow-wave sleep (SWS) was characterized by oscillations in the slow (<1 Hz) and delta (1-4 Hz) frequency range. The high-amplitude slow-wave complexes consisted in a positivity of depth LFP, associated with neuronal silence, followed by a sharp LFP negativity, correlated with an increase of firing. This pattern was of remarkable spatiotemporal coherence, because silences and increased firing occurred simultaneously in units recorded within a 7 mm distance in the cortex. During wake and rapid-eye-movement (REM) sleep, single units fired tonically, whereas LFPs displayed low-amplitude fast activities with increased power in fast frequencies (15-75 Hz). In contrast with the widespread synchronization during SWS, fast oscillations during REM and wake periods were synchronized only within neighboring electrodes and small time windows (100-500 msec). This local synchrony occurred in an apparent irregular manner, both spatially and temporally. Brief periods (<1 sec) of fast oscillations were also present during SWS in between slow-wave complexes. During these brief periods, the spatial and temporal coherence, as well as the relation between units and LFPs, was identical to that of fast oscillations of wake or REM sleep. These results show that natural SWS in cats is characterized by slow-wave complexes, synchronized over large cortical territories, interleaved with brief periods of fast oscillations, characterized by local synchrony, and of characteristics similar to that of the sustained fast oscillations of activated states.
Figures



References
-
- Achermann P, Borbély AA. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. - PubMed
-
- Achermann P, Borbély AA. Coherence analysis of the human sleep electroencephalogram. Neuroscience. 1998;85:1195–1208. - PubMed
-
- Amzica F, Steriade M. The K-complex: its slow (<1 Hz) rhythmicity and relation to delta waves. Neurology. 1997;49:952–959. - PubMed
-
- Ball CJ, Gloor P, Schaul N. The cortical electromicrophysiology of pathological delta waves in the electroencephalogram of cats. Electroencephalogr Clin Neurophysiol. 1977;43:346–361. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
