Differential expression of alpha1, alpha2, alpha3, and alpha5 GABAA receptor subunits in seizure-prone and seizure-resistant rat models of temporal lobe epilepsy
- PMID: 10341263
- PMCID: PMC6782587
- DOI: 10.1523/JNEUROSCI.19-11-04654.1999
Differential expression of alpha1, alpha2, alpha3, and alpha5 GABAA receptor subunits in seizure-prone and seizure-resistant rat models of temporal lobe epilepsy
Abstract
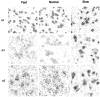
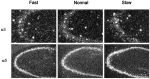
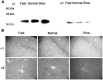
Temporal lobe epilepsy remains one of the most widespread seizure disorders in man, the etiology of which is controversial. Using new rat models of temporal lobe epilepsy that are either prone or resistant to develop complex partial seizures, we provide evidence that this seizure susceptibility may arise from arrested development of the GABAA receptor system. In seizure-prone (Fast kindling) and seizure-resistant (Slow kindling) rat models, both the mRNA and protein levels of the major alpha subunit expressed in adult brain (alpha1), as well as those highly expressed during development (alpha2, alpha3, and alpha5), were differentially expressed in both models compared with normal controls. We found that alpha1 subunit mRNA expression in the Fast kindling strain was approximately half the abundance of control rats, whereas in the Slow kindling strain, it was approximately 70% greater than that of controls. However, Fast rats overexpressed the alpha2, alpha3, and alpha5 ("embryonic") subunits, having a density 50-70% greater than controls depending on brain area, whereas the converse was true of Slow rats. Using subunit-specific antibodies to alpha1 and alpha5 subunits, quantitative immunoblots and immunocytochemistry revealed a concordance with the mRNA levels. alpha1 protein expression was approximately 50% less than controls in the Fast strain, whereas it was 200% greater in the Slow strain. In contrast, alpha5 subunit protein expression was greater in the Fast strain than either the control or Slow strain. These data suggest that a major predispositional factor in the development of temporal lobe epilepsy could be a failure to complete the normal switch from the GABAA receptor alpha subunits highly expressed during development (alpha2, alpha3, and alpha5) to those highly expressed in adulthood (alpha1).
Figures

Similar articles
-
Altered expression of GABA(A) and GABA(B) receptor subunit mRNAs in the hippocampus after kindling and electrically induced status epilepticus.Neuroscience. 2005;134(2):691-704. doi: 10.1016/j.neuroscience.2005.04.013. Neuroscience. 2005. PMID: 15951123
-
GABA(A) receptor subunits in the rat hippocampus III: altered messenger RNA expression in kainic acid-induced epilepsy.Neuroscience. 1997 Oct;80(4):1019-32. doi: 10.1016/s0306-4522(97)00144-9. Neuroscience. 1997. PMID: 9284057
-
Differential GABA(A) subunit expression following status epilepticus in seizure-prone and seizure-resistant rats: a putative mechanism for refractory drug response.Epilepsia. 2005;46 Suppl 5:3-9. doi: 10.1111/j.1528-1167.2005.01001.x. Epilepsia. 2005. PMID: 15987246
-
Genetically seizure-prone or seizure-resistant phenotypes and their associated behavioral comorbidities.Epilepsia. 2007;48 Suppl 9:30-2. doi: 10.1111/j.1528-1167.2007.01398.x. Epilepsia. 2007. PMID: 18047598 Review.
-
GABAA receptor subtype-selective modulators. II. α5-selective inverse agonists for cognition enhancement.Curr Top Med Chem. 2011;11(9):1203-14. doi: 10.2174/156802611795371314. Curr Top Med Chem. 2011. PMID: 21050171 Review.
Cited by
-
Hyperexcitability: From Normal Fear to Pathological Anxiety and Trauma.Front Syst Neurosci. 2022 Aug 4;16:727054. doi: 10.3389/fnsys.2022.727054. eCollection 2022. Front Syst Neurosci. 2022. PMID: 35993088 Free PMC article. Review.
-
Clinical Correlation of Altered Molecular Signatures in Epileptic Human Hippocampus and Amygdala.Mol Neurobiol. 2024 Feb;61(2):725-752. doi: 10.1007/s12035-023-03583-6. Epub 2023 Sep 2. Mol Neurobiol. 2024. PMID: 37658249 Free PMC article.
-
Making sense of nonsense GABA(A) receptor mutations associated with genetic epilepsies.Trends Mol Med. 2009 Sep;15(9):430-8. doi: 10.1016/j.molmed.2009.07.003. Epub 2009 Aug 31. Trends Mol Med. 2009. PMID: 19717338 Free PMC article. Review.
-
Animal models of limbic epilepsies: what can they tell us?Brain Pathol. 2002 Apr;12(2):240-56. doi: 10.1111/j.1750-3639.2002.tb00439.x. Brain Pathol. 2002. PMID: 11958378 Free PMC article. Review.
-
In the fast lane: Receptor trafficking during status epilepticus.Epilepsia Open. 2023 May;8 Suppl 1(Suppl 1):S35-S65. doi: 10.1002/epi4.12718. Epub 2023 Mar 20. Epilepsia Open. 2023. PMID: 36861477 Free PMC article. Review.
References
-
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. - PubMed
-
- Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. - PubMed
-
- Clark M, Massenburg GS, Weiss SR, Post RM. Analysis of the hippocampal GABAA receptor system in kindled rats by autoradiographic and in situ hybridization techniques: contingent tolerance to carbamazepine. Mol Brain Res. 1994;26:309–319. - PubMed
-
- Corcoran ME, Mason ST. Role of forebrain catecholamines in amygdaloid kindling. Brain Res. 1980;190:473–484. - PubMed
-
- Dailey JW, Reigel CE, Mishra PK, Jobe PC. Neurobiology of seizure predisposition in the genetically epilepsy-prone rat. Epilepsy Res. 1989;3:3–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
