Loss of transcription factor IRF-1 affects tumor susceptibility in mice carrying the Ha-ras transgene or nullizygosity for p53
- PMID: 10346812
- PMCID: PMC316726
- DOI: 10.1101/gad.13.10.1240
Loss of transcription factor IRF-1 affects tumor susceptibility in mice carrying the Ha-ras transgene or nullizygosity for p53
Abstract
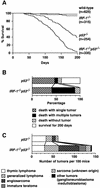
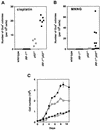
The transcription factor IRF-1 has been implicated in tumor suppression: IRF-1 suppresses cell transformation and mediates apoptosis in vitro. Here we show that the loss of IRF-1 alleles per se has no effect on spontaneous tumor development in the mouse but dramatically exacerbates previous tumor predispositions caused by the c-Ha-ras transgene or by nullizygosity for p53. Grossly altered tumor spectrum, as compared to p53-null mice, was also observed in mice lacking both IRF-1 and p53, and cells from these mice show significantly higher mutation rate. Our results suggest that IRF-1 is a new member of the tumor susceptibility genes.
Figures

Similar articles
-
Altered DNA repair and dysregulation of p53 in IRF-1 null hepatocytes.FASEB J. 1998 Feb;12(2):181-8. doi: 10.1096/fasebj.12.2.181. FASEB J. 1998. PMID: 9472983
-
IRF-1 reverts the transformed phenotype of oncogenically transformed cells in vitro and in vivo.Oncogene. 2003 Feb 20;22(7):1045-56. doi: 10.1038/sj.onc.1206260. Oncogene. 2003. PMID: 12592391
-
Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1.Cell. 1994 Jun 17;77(6):829-39. doi: 10.1016/0092-8674(94)90132-5. Cell. 1994. PMID: 8004672
-
IRF-1 as a negative regulator of cell proliferation.J Interferon Cytokine Res. 2002 Jan;22(1):39-47. doi: 10.1089/107999002753452647. J Interferon Cytokine Res. 2002. PMID: 11846974 Review.
-
Transcription factors IRF-1 and IRF-2: linking the immune responses and tumor suppression.J Cell Physiol. 1997 Nov;173(2):128-30. doi: 10.1002/(SICI)1097-4652(199711)173:2<128::AID-JCP8>3.0.CO;2-P. J Cell Physiol. 1997. PMID: 9365509 Review. No abstract available.
Cited by
-
Diverse mechanisms evolved by DNA viruses to inhibit early host defenses.Crit Rev Biochem Mol Biol. 2016 Nov/Dec;51(6):452-481. doi: 10.1080/10409238.2016.1226250. Epub 2016 Sep 21. Crit Rev Biochem Mol Biol. 2016. PMID: 27650455 Free PMC article. Review.
-
IFN-zeta/ limitin: a member of type I IFN with mild lympho-myelosuppression.J Cell Mol Med. 2005 Apr-Jun;9(2):244-54. doi: 10.1111/j.1582-4934.2005.tb00353.x. J Cell Mol Med. 2005. PMID: 15963247 Free PMC article. Review.
-
Genetic Programs Driving Oncogenic Transformation: Lessons from in Vitro Models.Int J Mol Sci. 2019 Dec 12;20(24):6283. doi: 10.3390/ijms20246283. Int J Mol Sci. 2019. PMID: 31842516 Free PMC article.
-
Frequent loss of heterozygosity at the interferon regulatory factor-1 gene locus in breast cancer.Breast Cancer Res Treat. 2010 May;121(1):227-31. doi: 10.1007/s10549-009-0509-8. Epub 2009 Aug 21. Breast Cancer Res Treat. 2010. PMID: 19697121 Free PMC article.
-
Tumor suppressor IRF-1 mediates retinoid and interferon anticancer signaling to death ligand TRAIL.EMBO J. 2004 Aug 4;23(15):3051-60. doi: 10.1038/sj.emboj.7600302. Epub 2004 Jul 8. EMBO J. 2004. PMID: 15241475 Free PMC article.
References
-
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. - PubMed
-
- Buckwalter MS, Lossie AC, Scarlett LM, Camper SA. Localization of the human chromosome 5q genes Gabra-1, Gabrg-2, Il-4, Il-5, and Irf-1 on mouse chromosome 11. Mamm Genome. 1992;3:604–607. - PubMed
-
- Demant P. Genetic resolution of susceptibility to cancer—New perspectives. Semin Cancer Biol. 1992;3:159–166. - PubMed
-
- Dietrich WF, Lander ES, Smith JS, Moser AR, Gould KA, Luongo C, Borenstein N, Dove W. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993;75:631–639. - PubMed
-
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
