Transcriptional control of ADH genes in the xylose-fermenting yeast Pichia stipitis
- PMID: 10347014
- PMCID: PMC91349
- DOI: 10.1128/AEM.65.6.2363-2368.1999
Transcriptional control of ADH genes in the xylose-fermenting yeast Pichia stipitis
Abstract
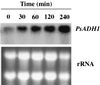
We studied the expression of the genes encoding group I alcohol dehydrogenases (PsADH1 and PsADH2) in the xylose-fermenting yeast Pichia stipitis CBS 6054. The cells expressed PsADH1 approximately 10 times higher under oxygen-limited conditions than under fully aerobic conditions when cultivated on xylose. Transcripts of PsADH2 were not detectable under either aeration condition. We used a PsADH1::lacZ fusion to monitor PsADH1 expression and found that expression increased as oxygen decreased. The level of PsADH1 transcript was repressed about 10-fold in cells grown in the presence of heme under oxygen-limited conditions. Concomitantly with the induction of PsADH1, PsCYC1 expression was repressed. These results indicate that oxygen availability regulates PsADH1 expression and that regulation may be mediated by heme. The regulation of PsADH2 expression was also examined in other genetic backgrounds. Disruption of PsADH1 dramatically increased PsADH2 expression on nonfermentable carbon sources under fully aerobic conditions, indicating that the expression of PsADH2 is subject to feedback regulation under these conditions.
Figures




Similar articles
-
Pichia stipitis genes for alcohol dehydrogenase with fermentative and respiratory functions.Appl Environ Microbiol. 1998 Apr;64(4):1350-8. doi: 10.1128/AEM.64.4.1350-1358.1998. Appl Environ Microbiol. 1998. PMID: 9546172 Free PMC article.
-
Molecular cloning of alcohol dehydrogenase genes of the yeast Pichia stipitis and identification of the fermentative ADH.Yeast. 1998 Oct;14(14):1311-25. doi: 10.1002/(SICI)1097-0061(1998100)14:14<1311::AID-YEA315>3.0.CO;2-T. Yeast. 1998. PMID: 9802210
-
Increased xylose reductase activity in the xylose-fermenting yeast Pichia stipitis by overexpression of XYL1.Appl Biochem Biotechnol. 1996 Spring;57-58:267-76. doi: 10.1007/978-1-4612-0223-3_24. Appl Biochem Biotechnol. 1996. PMID: 8669900
-
Expression of bacterial hemoglobin in the yeast, Pichia pastoris, with a low O2-induced promoter.Biotechnol Lett. 2005 Oct;27(19):1491-7. doi: 10.1007/s10529-005-1324-x. Biotechnol Lett. 2005. PMID: 16231222
-
Conversion of xylose to ethanol by recombinant Saccharomyces cerevisiae: importance of xylulokinase (XKS1) and oxygen availability.Metab Eng. 2001 Jul;3(3):236-49. doi: 10.1006/mben.2000.0191. Metab Eng. 2001. PMID: 11461146
Cited by
-
Temporal analysis of xylose fermentation by Scheffersomyces stipitis using shotgun proteomics.J Ind Microbiol Biotechnol. 2012 Oct;39(10):1507-14. doi: 10.1007/s10295-012-1147-4. Epub 2012 May 26. J Ind Microbiol Biotechnol. 2012. PMID: 22638791
-
Transcriptional regulation of the gene encoding an alcohol dehydrogenase in the archaeon Sulfolobus solfataricus involves multiple factors and control elements.J Bacteriol. 2003 Jul;185(13):3926-34. doi: 10.1128/JB.185.13.3926-3934.2003. J Bacteriol. 2003. PMID: 12813087 Free PMC article.
-
Pichia stipitis genomics, transcriptomics, and gene clusters.FEMS Yeast Res. 2009 Sep;9(6):793-807. doi: 10.1111/j.1567-1364.2009.00525.x. Epub 2009 Apr 27. FEMS Yeast Res. 2009. PMID: 19659741 Free PMC article. Review.
-
Effects of glucose, ethanol and acetic acid on regulation of ADH2 gene from Lachancea fermentati.PeerJ. 2016 Mar 10;4:e1751. doi: 10.7717/peerj.1751. eCollection 2016. PeerJ. 2016. PMID: 26989608 Free PMC article.
-
Identification of the ADH gene family in Trichosporon asahii and the role of TaADH_like in pathogenicity and fluconazole resistance.BMC Genomics. 2025 Apr 7;26(1):352. doi: 10.1186/s12864-025-11546-5. BMC Genomics. 2025. PMID: 40197151 Free PMC article.
References
-
- Bennetzen J L, Hall B D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase I. J Biol Chem. 1982;257:3018–3025. - PubMed
-
- Bergmeyer H U. Methods of enzymatic analysis. 3rd ed. Vol. 11. Weinheim, Germany: Verlag Chemie; 1983. p. 139.
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

