Analysis of fungal diversity in the wheat rhizosphere by sequencing of cloned PCR-amplified genes encoding 18S rRNA and temperature gradient gel electrophoresis
- PMID: 10347051
- PMCID: PMC91386
- DOI: 10.1128/AEM.65.6.2614-2621.1999
Analysis of fungal diversity in the wheat rhizosphere by sequencing of cloned PCR-amplified genes encoding 18S rRNA and temperature gradient gel electrophoresis
Abstract
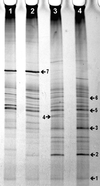
Like bacteria, fungi play an important role in the soil ecosystem. As only a small fraction of the fungi present in soil can be cultured, conventional microbiological techniques yield only limited information on the composition and dynamics of fungal communities in soil. DNA-based methods do not depend on the culturability of microorganisms, and therefore they offer an attractive alternative for the study of complex fungal community structures. For this purpose, we designed various PCR primers that allow the specific amplification of fungal 18S-ribosomal-DNA (rDNA) sequences, even in the presence of nonfungal 18S rDNA. DNA was extracted from the wheat rhizosphere, and 18S rDNA gene banks were constructed in Escherichia coli by cloning PCR products generated with primer pairs EF4-EF3 (1. 4 kb) and EF4-fung5 (0.5 kb). Fragments of 0.5 kb from the cloned inserts were sequenced and compared to known rDNA sequences. Sequences from all major fungal taxa were amplified by using both primer pairs. As predicted by computer analysis, primer pair EF4-EF3 appeared slightly biased to amplify Basidiomycota and Zygomycota, whereas EF4-fung5 amplified mainly Ascomycota. The 61 clones that were sequenced matched the sequences of 24 different species in the Ribosomal Database Project (RDP) database. Similarity values ranged from 0.676 to 1. Temperature gradient gel electrophoresis (TGGE) analysis of the fungal community in the wheat rhizosphere of a microcosm experiment was carried out after amplification of total DNA with both primer pairs. This resulted in reproducible, distinctive fingerprints, confirming the difference in amplification specificity. Clear banding patterns were obtained with soil and rhizosphere samples by using both primer sets in combination. By comparing the electrophoretic mobility of community fingerprint bands to that of the bands obtained with separate clones, some could be tentatively identified. While 18S-rDNA sequences do not always provide the taxonomic resolution to identify fungal species and strains, they do provide information on the diversity and dynamics of groups of related species in environmental samples with sufficient resolution to produce discrete bands which can be separated by TGGE. This combination of 18S-rDNA PCR amplification and TGGE community analysis should allow study of the diversity, composition, and dynamics of the fungal community in bulk soil and in the rhizosphere.
Figures


Similar articles
-
Potential bias of fungal 18S rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil.Environ Microbiol. 2003 Jan;5(1):36-47. doi: 10.1046/j.1462-2920.2003.00383.x. Environ Microbiol. 2003. PMID: 12542711
-
Soil fungal communities underneath willow canopies on a primary successional glacier forefront: rDNA sequence results can be affected by primer selection and chimeric data.Microb Ecol. 2007 Feb;53(2):233-46. doi: 10.1007/s00248-004-0006-x. Microb Ecol. 2007. PMID: 17106807
-
Detection and characterization of fungal infections of Ammophila arenaria (marram grass) roots by denaturing gradient gel electrophoresis of specifically amplified 18s rDNA.Appl Environ Microbiol. 1997 Oct;63(10):3858-65. doi: 10.1128/aem.63.10.3858-3865.1997. Appl Environ Microbiol. 1997. PMID: 9327549 Free PMC article.
-
Fungal community analysis by high-throughput sequencing of amplified markers--a user's guide.New Phytol. 2013 Jul;199(1):288-299. doi: 10.1111/nph.12243. Epub 2013 Mar 28. New Phytol. 2013. PMID: 23534863 Free PMC article. Review.
-
16S, 18S gene, and ITS region Sequencing: Expanded Applications in Pathology Diagnostics and Research.Cesk Patol. 2025;60(4):176-180. Cesk Patol. 2025. PMID: 40088441 Review. English.
Cited by
-
Vertical niche differentiation by hyphae of ectomycorrhizal fungi in soil.New Phytol. 2002 Dec;156(3):323-325. doi: 10.1046/j.1469-8137.2002.00548.x. New Phytol. 2002. PMID: 33873565 No abstract available.
-
Characterization of bacterial and fungal soil communities by automated ribosomal intergenic spacer analysis fingerprints: biological and methodological variability.Appl Environ Microbiol. 2001 Oct;67(10):4479-87. doi: 10.1128/AEM.67.10.4479-4487.2001. Appl Environ Microbiol. 2001. PMID: 11571146 Free PMC article.
-
Selective foraging of fungi by collembolans in soil.Biol Lett. 2005 Jun 22;1(2):243-6. doi: 10.1098/rsbl.2004.0286. Biol Lett. 2005. PMID: 17148177 Free PMC article.
-
Oligonucleotide fingerprinting of rRNA genes for analysis of fungal community composition.Appl Environ Microbiol. 2002 Dec;68(12):5999-6004. doi: 10.1128/AEM.68.12.5999-6004.2002. Appl Environ Microbiol. 2002. PMID: 12450821 Free PMC article.
-
Metagenomic and small-subunit rRNA analyses reveal the genetic diversity of bacteria, archaea, fungi, and viruses in soil.Appl Environ Microbiol. 2007 Nov;73(21):7059-66. doi: 10.1128/AEM.00358-07. Epub 2007 Sep 7. Appl Environ Microbiol. 2007. PMID: 17827313 Free PMC article.
References
-
- Alabouvette C. Biological control of Fusarium wilt pathogens in suppressive soils. In: Hornby D, editor. Biological control of soil-borne pathogens. Wallingford, United Kingdom: CAB International; 1990. pp. 27–34.
-
- Bock M, Maiwald M, Kappe R, Näher H. Polymerase chain reaction-based detection of dermatophyte DNA with a fungus-specific primer system. Mycoses. 1994;37:79–84. - PubMed
-
- Christensen M. Species diversity and dominance in fungal communities. In: Wicklow D T, Carroll G C, editors. The fungal community. New York, N.Y: Marcel Dekker, Inc.; 1981. pp. 201–232.
-
- De Ley F A A M, Lynch J M. Functional diversity of the rhizosphere. In: Ogoshi A, Kobayashi K, Homma Y, Kadoma F, Kondo N, Akino S, editors. Plant growth-promoting rhizobacteria. Proceedings of the Fourth International Workshop on plant-growth promoting rhizobacteria. Paris, France: OECD; 1997. pp. 38–43.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

