Peptide antibiotics
- PMID: 10348745
- PMCID: PMC89271
- DOI: 10.1128/AAC.43.6.1317
Peptide antibiotics
Figures

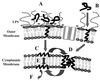
References
-
- Abee T. Pore-forming bacteriocins of gram-positive bacteria and self-protection mechanisms of producer organisms. FEMS Microbiol Lett. 1995;129:1–10. - PubMed
-
- Baba T, Schneewind O. Instruments of microbial warfare: bacteriocin synthesis, toxicity and immunity. Trends Microbiol. 1998;6:66–71. - PubMed
-
- Bello J, Bello H R, Granados E. Conformation and aggregation of melittin: dependence on pH and concentration. Biochemistry. 1982;21:461–465. - PubMed
-
- Bessalle R, Kapitkovsky A, Gorea A, Shalit I, Fridkin M. All-d-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 1990;274:151–155. - PubMed
-
- Bessalle R, Gorea A, Shalit I, Metzger J W, Dass C, Desiderio D M. Structure-function studies of amphiphilic antibacterial peptides. J Med Chem. 1993;36:1203–1209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

