Maltose metabolism in the hyperthermophilic archaeon Thermococcus litoralis: purification and characterization of key enzymes
- PMID: 10348846
- PMCID: PMC93801
- DOI: 10.1128/JB.181.11.3358-3367.1999
Maltose metabolism in the hyperthermophilic archaeon Thermococcus litoralis: purification and characterization of key enzymes
Abstract
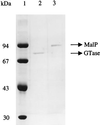
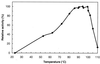
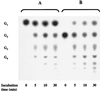
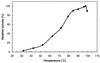
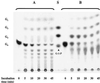
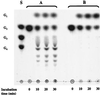
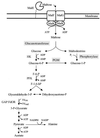
Maltose metabolism was investigated in the hyperthermophilic archaeon Thermococcus litoralis. Maltose was degraded by the concerted action of 4-alpha-glucanotransferase and maltodextrin phosphorylase (MalP). The first enzyme produced glucose and a series of maltodextrins that could be acted upon by MalP when the chain length of glucose residues was equal or higher than four, to produce glucose-1-phosphate. Phosphoglucomutase activity was also detected in T. litoralis cell extracts. Glucose derived from the action of 4-alpha-glucanotransferase was subsequently metabolized via an Embden-Meyerhof pathway. The closely related organism Pyrococcus furiosus used a different metabolic strategy in which maltose was cleaved primarily by the action of an alpha-glucosidase, a p-nitrophenyl-alpha-D-glucopyranoside (PNPG)-hydrolyzing enzyme, producing glucose from maltose. A PNPG-hydrolyzing activity was also detected in T. litoralis, but maltose was not a substrate for this enzyme. The two key enzymes in the pathway for maltose catabolism in T. litoralis were purified to homogeneity and characterized; they were constitutively synthesized, although phosphorylase expression was twofold induced by maltodextrins or maltose. The gene encoding MalP was obtained by complementation in Escherichia coli and sequenced (calculated molecular mass, 96,622 Da). The enzyme purified from the organism had a specific activity for maltoheptaose, at the temperature for maximal activity (98 degrees C), of 66 U/mg. A Km of 0.46 mM was determined with heptaose as the substrate at 60 degrees C. The deduced amino acid sequence had a high degree of identity with that of the putative enzyme from the hyperthermophilic archaeon Pyrococcus horikoshii OT3 (66%) and with sequences of the enzymes from the hyperthermophilic bacterium Thermotoga maritima (60%) and Mycobacterium tuberculosis (31%) but not with that of the enzyme from E. coli (13%). The consensus binding site for pyridoxal 5'-phosphate is conserved in the T. litoralis enzyme.
Figures


References
-
- Bibel M, Brettl C, Gosslar U, Kriegshäuser G, Liebl W. Isolation and analysis of genes for amylolytic enzymes of the hyperthermophilic bacterium Thermotoga maritima. FEMS Microbiol Lett. 1998;158:9–15. - PubMed
-
- Boeck B, Schinzel R. Purification and characterisation of an α-glucan phosphorylase from the thermophilic bacterium Thermus thermophilus. Eur J Biochem. 1996;239:150–155. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

