Cd(II)-responsive and constitutive mutants implicate a novel domain in MerR
- PMID: 10348859
- PMCID: PMC93814
- DOI: 10.1128/JB.181.11.3462-3471.1999
Cd(II)-responsive and constitutive mutants implicate a novel domain in MerR
Abstract
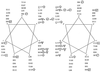
Expression of the Tn21 mercury resistance (mer) operon is controlled by a metal-sensing repressor-activator, MerR. When present, MerR always binds to the same position on the DNA (the operator merO), repressing transcription of the structural genes merTPCAD in the absence of Hg(II) and inducing their transcription in the presence of Hg(II). Although it has two potential binding sites, the purified MerR homodimer binds only one Hg(II) ion, employing Cys82 from one monomer and Cys117 and Cys126 from the other. When MerR binds Hg(II), it changes allosterically and also distorts the merO DNA to facilitate transcriptional initiation by sigma70 RNA polymerase. Wild-type MerR is highly specific for Hg(II) and is 100- and 1, 000-fold less responsive to the chemically related group 12 metals, Cd(II) and Zn(II), respectively. We sought merR mutants that respond to Cd(II) and obtained 11 Cd(II)-responsive and 5 constitutive mutants. The Cd(II)-responsive mutants, most of which had only single-residue replacements, were also repression deficient and still Hg(II) responsive but, like the wild type, were completely unresponsive to Zn(II). None of the Cd(II)-responsive mutations occurred in the DNA binding domain or replaced any of the key Cys residues. Five Cd(II)-responsive single mutations lie in the antiparallel coiled-coil domain between Cys82 and Cys117 which constitutes the dimer interface. These mutations identify 10 new positions whose alteration significantly affect MerR's metal responsiveness or its repressor function. They give rise to specific predictions for how MerR distinguishes group 12 metals, and they refine our model of the novel domain structure of MerR. Secondary-structure predictions suggest that certain elements of this model also apply to other MerR family regulators.
Figures

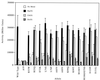
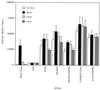
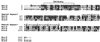

 , constitutive mutants constructed in an A89V or S131L background (R62Q and V124A individually combined with S131L, others each with A89V). Superscripted numbers indicate sets of double mutations; e.g., set 2 includes V109E and V124A.
, constitutive mutants constructed in an A89V or S131L background (R62Q and V124A individually combined with S131L, others each with A89V). Superscripted numbers indicate sets of double mutations; e.g., set 2 includes V109E and V124A.
Similar articles
-
Engineered single-chain, antiparallel, coiled coil mimics the MerR metal binding site.J Bacteriol. 2004 Mar;186(6):1861-8. doi: 10.1128/JB.186.6.1861-1868.2004. J Bacteriol. 2004. PMID: 14996817 Free PMC article.
-
Cd-specific mutants of mercury-sensing regulatory protein MerR, generated by directed evolution.Appl Environ Microbiol. 2011 Sep;77(17):6215-24. doi: 10.1128/AEM.00662-11. Epub 2011 Jul 15. Appl Environ Microbiol. 2011. PMID: 21764963 Free PMC article.
-
In vivo DNA-protein interactions at the divergent mercury resistance (mer) promoters. II. Repressor/activator (MerR)-RNA polymerase interaction with merOP mutants.J Biol Chem. 1993 Feb 5;268(4):2632-9. J Biol Chem. 1993. PMID: 8428940
-
Bacterial resistances to inorganic mercury salts and organomercurials.Plasmid. 1992 Jan;27(1):4-16. doi: 10.1016/0147-619x(92)90002-r. Plasmid. 1992. PMID: 1311113 Review.
-
The MerR family of transcriptional regulators.FEMS Microbiol Rev. 2003 Jun;27(2-3):145-63. doi: 10.1016/S0168-6445(03)00051-2. FEMS Microbiol Rev. 2003. PMID: 12829265 Review.
Cited by
-
Inducible metabolism of phenolic acids in Pediococcus pentosaceus is encoded by an autoregulated operon which involves a new class of negative transcriptional regulator.J Bacteriol. 2000 Dec;182(23):6724-31. doi: 10.1128/JB.182.23.6724-6731.2000. J Bacteriol. 2000. PMID: 11073918 Free PMC article.
-
Transcription-defective soxR mutants of Escherichia coli: isolation and in vivo characterization.J Bacteriol. 2003 Apr;185(8):2441-50. doi: 10.1128/JB.185.8.2441-2450.2003. J Bacteriol. 2003. PMID: 12670967 Free PMC article.
-
Structural Analysis of the Hg(II)-Regulatory Protein Tn501 MerR from Pseudomonas aeruginosa.Sci Rep. 2016 Sep 19;6:33391. doi: 10.1038/srep33391. Sci Rep. 2016. PMID: 27641146 Free PMC article.
-
Effect of pH on intracellular accumulation of trace concentrations of Hg(II) in Escherichia coli under anaerobic conditions, as measured using a mer-lux bioreporter.Appl Environ Microbiol. 2008 Feb;74(3):667-75. doi: 10.1128/AEM.00717-07. Epub 2007 Dec 14. Appl Environ Microbiol. 2008. PMID: 18083863 Free PMC article.
-
Bacterial Metallostasis: Metal Sensing, Metalloproteome Remodeling, and Metal Trafficking.Chem Rev. 2024 Dec 25;124(24):13574-13659. doi: 10.1021/acs.chemrev.4c00264. Epub 2024 Dec 10. Chem Rev. 2024. PMID: 39658019 Free PMC article. Review.
References
-
- Ahmed M, Borsch C M, Taylor S S, Vázquez-Laslop N, Neyfakh A A. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J Biol Chem. 1994;269:28506–28513. - PubMed
-
- Ansari A Z, Bradner J E, O’Halloran T V. DNA-bend modulation in a repressor-to-activator switching mechanism. Nature. 1995;374:371–375. - PubMed
-
- Ansari A Z, Chael M L, O’Halloran T V. Allosteric underwinding of DNA is a critical step in positive control of transcription by Hg-MerR. Nature. 1992;355:87–89. - PubMed
-
- Atwell S, Ultsch M, De Vos A M, Wells J A. Structural plasticity in a remodeled protein-protein interface. Science. 1997;278:1125–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

