Function of proline residues of MotA in torque generation by the flagellar motor of Escherichia coli
- PMID: 10348868
- PMCID: PMC93823
- DOI: 10.1128/JB.181.11.3542-3551.1999
Function of proline residues of MotA in torque generation by the flagellar motor of Escherichia coli
Abstract
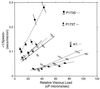
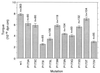
Bacterial flagellar motors obtain energy for rotation from the membrane gradient of protons or, in some species, sodium ions. The molecular mechanism of flagellar rotation is not understood. MotA and MotB are integral membrane proteins that function in proton conduction and are believed to form the stator of the motor. Previous mutational studies identified two conserved proline residues in MotA (Pro 173 and Pro 222 in the protein from Escherichia coli) and a conserved aspartic acid residue in MotB (Asp 32) that are important for function. Asp 32 of MotB probably forms part of the proton path through the motor. To learn more about the roles of the conserved proline residues of MotA, we examined motor function in Pro 173 and Pro 222 mutants, making measurements of torque at high load, speed at low and intermediate loads, and solvent-isotope effects (D2O versus H2O). Proton conduction by wild-type and mutant MotA-MotB channels was also assayed, by a growth defect that occurs upon overexpression. Several different mutations of Pro 173 reduced the torque of the motor under high load, and a few prevented motor rotation but still allowed proton flow through the MotA-MotB channels. These and other properties of the mutants suggest that Pro 173 has a pivotal role in coupling proton flow to motor rotation and is positioned in the channel near Asp 32 of MotB. Replacements of Pro 222 abolished function in all assays and were strongly dominant. Certain Pro 222 mutant proteins prevented swimming almost completely when expressed at moderate levels in wild-type cells. This dominance might be caused by rotor-stator jamming, because it was weaker when FliG carried a mutation believed to increase rotor-stator clearance. We propose a mechanism for torque generation, in which specific functions are suggested for the proline residues of MotA and Asp32 of MotB.
Figures
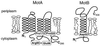

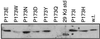




Similar articles
-
Function of protonatable residues in the flagellar motor of Escherichia coli: a critical role for Asp 32 of MotB.J Bacteriol. 1998 May;180(10):2729-35. doi: 10.1128/JB.180.10.2729-2735.1998. J Bacteriol. 1998. PMID: 9573160 Free PMC article.
-
GFP Fusion to the N-Terminus of MotB Affects the Proton Channel Activity of the Bacterial Flagellar Motor in Salmonella.Biomolecules. 2020 Aug 29;10(9):1255. doi: 10.3390/biom10091255. Biomolecules. 2020. PMID: 32872412 Free PMC article.
-
Conformational change in the stator of the bacterial flagellar motor.Biochemistry. 2001 Oct 30;40(43):13041-50. doi: 10.1021/bi011263o. Biochemistry. 2001. PMID: 11669642
-
Flagellar movement driven by proton translocation.FEBS Lett. 2003 Jun 12;545(1):86-95. doi: 10.1016/s0014-5793(03)00397-1. FEBS Lett. 2003. PMID: 12788496 Review.
-
Structure and function of the bi-directional bacterial flagellar motor.Biomolecules. 2014 Feb 18;4(1):217-34. doi: 10.3390/biom4010217. Biomolecules. 2014. PMID: 24970213 Free PMC article. Review.
Cited by
-
Gate-controlled proton diffusion and protonation-induced ratchet motion in the stator of the bacterial flagellar motor.Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):7737-42. doi: 10.1073/pnas.1502991112. Epub 2015 Jun 8. Proc Natl Acad Sci U S A. 2015. PMID: 26056313 Free PMC article.
-
Membrane segment organization in the stator complex of the flagellar motor: implications for proton flow and proton-induced conformational change.Biochemistry. 2008 Oct 28;47(43):11332-9. doi: 10.1021/bi801347a. Epub 2008 Oct 4. Biochemistry. 2008. PMID: 18834143 Free PMC article.
-
Insertional inactivation of genes encoding components of the sodium-type flagellar motor and switch of Vibrio parahaemolyticus.J Bacteriol. 2000 Feb;182(4):1035-45. doi: 10.1128/JB.182.4.1035-1045.2000. J Bacteriol. 2000. PMID: 10648530 Free PMC article.
-
Expression, purification and biochemical characterization of the cytoplasmic loop of PomA, a stator component of the Na(+) driven flagellar motor.Biophysics (Nagoya-shi). 2013 Feb 5;9:21-9. doi: 10.2142/biophysics.9.21. eCollection 2013. Biophysics (Nagoya-shi). 2013. PMID: 27493537 Free PMC article.
-
Pseudomonas aeruginosa evasion of phagocytosis is mediated by loss of swimming motility and is independent of flagellum expression.Infect Immun. 2010 Jul;78(7):2937-45. doi: 10.1128/IAI.00144-10. Epub 2010 May 10. Infect Immun. 2010. PMID: 20457788 Free PMC article.
References
-
- Blair D F. How bacteria sense and swim. Annu Rev Microbiol. 1995;49:489–522. - PubMed
-
- Blair D F, Berg H C. Restoration of torque in defective flagellar motors. Science. 1988;242:1678–1681. - PubMed
-
- Blair D F, Berg H C. The MotA protein of E. coli is a proton-conducting component of the flagellar motor. Cell. 1990;60:439–449. - PubMed
-
- Blair D F, Berg H C. Mutations in the MotA protein of Escherichia coli reveal domains critical for proton conduction. J Mol Biol. 1991;221:1433–1442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

