NH2-Terminal targeting motifs direct dual specificity A-kinase-anchoring protein 1 (D-AKAP1) to either mitochondria or endoplasmic reticulum
- PMID: 10352013
- PMCID: PMC2133123
- DOI: 10.1083/jcb.145.5.951
NH2-Terminal targeting motifs direct dual specificity A-kinase-anchoring protein 1 (D-AKAP1) to either mitochondria or endoplasmic reticulum
Abstract
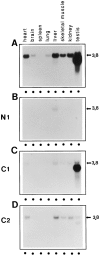
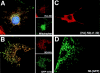
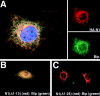
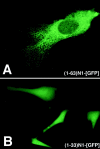
Subcellular localization directed by specific targeting motifs is an emerging theme for regulating signal transduction pathways. For cAMP-dependent protein kinase (PKA), this is achieved primarily by its association with A-kinase-anchoring proteins (AKAPs). Dual specificity AKAP1, (D-AKAP1) binds to both type I and type II regulatory subunits and has two NH2-terminal (N0 and N1) and two COOH-terminal (C1 and C2) splice variants (. J. Biol. Chem. 272:8057). Here we report that the splice variants of D-AKAP1 are expressed in a tissue-specific manner with the NH2-terminal motifs serving as switches to localize D-AKAP1 at different sites. Northern blots showed that the N1 splice is expressed primarily in liver, while the C1 splice is predominant in testis. The C2 splice shows a general expression pattern. Microinjecting expression constructs of D-AKAP1(N0) epitope-tagged at either the NH2 or the COOH terminus showed their localization to the mitochondria based on immunocytochemistry. Deletion of N0(1-30) abolished mitochondrial targeting while N0(1-30)-GFP localized to mitochondria. Residues 1-30 of N0 are therefore necessary and sufficient for mitochondria targeting. Addition of the 33 residues of N1 targets D-AKAP1 to the ER and residues 1-63 fused to GFP are necessary and sufficient for ER targeting. Residues 14-33 of N1 are especially important for targeting to ER; however, residues 1-33 alone fused to GFP gave a diffuse distribution. N1(14-33) thus serves two functions: (a) it suppresses the mitochondrial-targeting motif located within residues 1-30 of N0 and (b) it exposes an ER-targeting motif that is at least partially contained within the N0(1-30) motif. This represents the first example of a differentially targeted AKAP and adds an additional level of complexity to the PKA signaling network.
Figures





Similar articles
-
A 15-residue bifunctional element in D-AKAP1 is required for both endoplasmic reticulum and mitochondrial targeting.J Biol Chem. 2002 Jul 26;277(30):27328-36. doi: 10.1074/jbc.M201421200. Epub 2002 May 6. J Biol Chem. 2002. PMID: 11994283
-
Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits.J Biol Chem. 1997 Mar 21;272(12):8057-64. doi: 10.1074/jbc.272.12.8057. J Biol Chem. 1997. PMID: 9065479
-
D-AKAP2, a novel protein kinase A anchoring protein with a putative RGS domain.Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11184-9. doi: 10.1073/pnas.94.21.11184. Proc Natl Acad Sci U S A. 1997. PMID: 9326583 Free PMC article.
-
The biological functions of A-kinase anchor proteins.J Mol Biol. 2001 Apr 27;308(2):99-114. doi: 10.1006/jmbi.2001.4585. J Mol Biol. 2001. PMID: 11327755 Review.
-
A-kinase anchoring protein 1 (AKAP1) and its role in some cardiovascular diseases.J Mol Cell Cardiol. 2020 Jan;138:99-109. doi: 10.1016/j.yjmcc.2019.11.154. Epub 2019 Nov 26. J Mol Cell Cardiol. 2020. PMID: 31783032 Review.
Cited by
-
Pancortin-2 interacts with WAVE1 and Bcl-xL in a mitochondria-associated protein complex that mediates ischemic neuronal death.J Neurosci. 2007 Feb 14;27(7):1519-28. doi: 10.1523/JNEUROSCI.5154-06.2007. J Neurosci. 2007. PMID: 17301160 Free PMC article.
-
Mitochondria signaling pathways in allergic asthma.J Investig Med. 2022 Apr;70(4):863-882. doi: 10.1136/jim-2021-002098. Epub 2022 Feb 15. J Investig Med. 2022. PMID: 35168999 Free PMC article. Review.
-
PKA, PKC, and AKAP localization in and around the neuromuscular junction.BMC Neurosci. 2001;2:17. doi: 10.1186/1471-2202-2-17. Epub 2001 Oct 23. BMC Neurosci. 2001. PMID: 11716788 Free PMC article.
-
Functional characterization of a mitochondrial Ser/Thr protein phosphatase in cell death regulation.Methods Enzymol. 2009;457:255-73. doi: 10.1016/S0076-6879(09)05014-9. Methods Enzymol. 2009. PMID: 19426872 Free PMC article.
-
Potential for therapeutic targeting of AKAP signaling complexes in nervous system disorders.Pharmacol Ther. 2018 May;185:99-121. doi: 10.1016/j.pharmthera.2017.12.004. Epub 2017 Dec 17. Pharmacol Ther. 2018. PMID: 29262295 Free PMC article. Review.
References
-
- Andres DA, Dickerson IM, Dixon JE. Variants of the carboxyl-terminal KDEL sequence direct intracellular retention. J Biol Chem. 1990;265:5952–5955. - PubMed
-
- Chen Q, Lin RY, Rubin CS. Organelle-specific targeting of protein kinase AII (PKAII) J Biol Chem. 1997;272:15247–15257. - PubMed
-
- Coghlan V, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WH, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. - PubMed
-
- Feliciello A, Rubin CS, Avvedimento EV, Gottesman ME. Expression of a kinase anchor protein 121 is regulated by hormones in thyroid and testicular germ cells. J Biol Chem. 1998;273:23361–23366. - PubMed
-
- Haas IG. BiP (GRP78), an essential hsp70 resident protein in the endoplasmic reticulum. Experientia. 1994;50:1012–1020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

