Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia
- PMID: 10352018
- PMCID: PMC2133125
- DOI: 10.1083/jcb.145.5.1009
Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia
Abstract
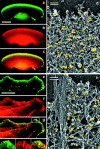
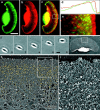
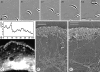
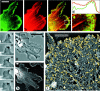
The leading edge (approximately 1 microgram) of lamellipodia in Xenopus laevis keratocytes and fibroblasts was shown to have an extensively branched organization of actin filaments, which we term the dendritic brush. Pointed ends of individual filaments were located at Y-junctions, where the Arp2/3 complex was also localized, suggesting a role of the Arp2/3 complex in branch formation. Differential depolymerization experiments suggested that the Arp2/3 complex also provided protection of pointed ends from depolymerization. Actin depolymerizing factor (ADF)/cofilin was excluded from the distal 0.4 micrometer++ of the lamellipodial network of keratocytes and in fibroblasts it was located within the depolymerization-resistant zone. These results suggest that ADF/cofilin, per se, is not sufficient for actin brush depolymerization and a regulatory step is required. Our evidence supports a dendritic nucleation model (Mullins, R.D., J.A. Heuser, and T.D. Pollard. 1998. Proc. Natl. Acad. Sci. USA. 95:6181-6186) for lamellipodial protrusion, which involves treadmilling of a branched actin array instead of treadmilling of individual filaments. In this model, Arp2/3 complex and ADF/cofilin have antagonistic activities. Arp2/3 complex is responsible for integration of nascent actin filaments into the actin network at the cell front and stabilizing pointed ends from depolymerization, while ADF/cofilin promotes filament disassembly at the rear of the brush, presumably by pointed end depolymerization after dissociation of the Arp2/3 complex.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

