Cyclic expression of endothelin-converting enzyme-1 mediates the functional regulation of seminiferous tubule contraction
- PMID: 10352019
- PMCID: PMC2133129
- DOI: 10.1083/jcb.145.5.1027
Cyclic expression of endothelin-converting enzyme-1 mediates the functional regulation of seminiferous tubule contraction
Abstract
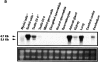
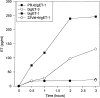
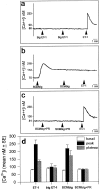
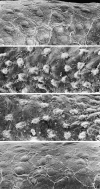
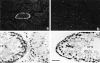
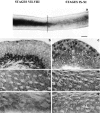
The potent smooth muscle agonist endothelin-1 (ET-1) is involved in the local control of seminiferous tubule contractility, which results in the forward propulsion of tubular fluid and spermatozoa, through its action on peritubular myoid cells. ET-1, known to be produced in the seminiferous epithelium by Sertoli cells, is derived from the inactive intermediate big endothelin-1 (big ET-1) through a specific cleavage operated by the endothelin-converting enzyme (ECE), a membrane-bound metalloprotease with ectoenzymatic activity. The data presented suggest that the timing of seminiferous tubule contractility is controlled locally by the cyclic interplay between different cell types. We have studied the expression of ECE by Sertoli cells and used myoid cell cultures and seminiferous tubule explants to monitor the biological activity of the enzymatic reaction product. Northern blot analysis showed that ECE-1 (and not ECE-2) is specifically expressed in Sertoli cells; competitive enzyme immunoassay of ET production showed that Sertoli cell monolayers are capable of cleaving big ET-1, an activity inhibited by the ECE inhibitor phosphoramidon. Microfluorimetric analysis of intracellular calcium mobilization in single cells showed that myoid cells do not respond to big endothelin, nor to Sertoli cell plain medium, but to the medium conditioned by Sertoli cells in the presence of big ET-1, resulting in cell contraction and desensitization to further ET-1 stimulation; in situ hybridization analysis shows regional differences in ECE expression, suggesting that pulsatile production of endothelin by Sertoli cells (at specific "stages" of the seminiferous epithelium) may regulate the cyclicity of tubular contraction; when viewed in a scanning electron microscope, segments of seminiferous tubules containing the specific stages characterized by high expression of ECE were observed to contract in response to big ET-1, whereas stages with low ECE expression remained virtually unaffected. These data indicate that endothelin-mediated spatiotemporal control of rhythmic tubular contractility might be operated by Sertoli cells through the cyclic expression of ECE-1, which is, in turn, dependent upon the timing of spermatogenesis.
Figures




References
-
- Ackermann A. Substituted naphtol AS phosphate derivatives for the localization of leukocytes alkaline phosphatase activity. Lab Invest. 1962;11:563–566. - PubMed
-
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. - PubMed
-
- Bardin, C.W., C. Yan Cheng, N.A. Musto, and G.L. Gunsalus. 1988. The Sertoli cell. In The Physiology of Reproduction. E. Knobil and J. Neill, editors. Raven Press, Ltd., New York. 1:933–974.
-
- Barnes K, Brown C, Turner AJ. Endothelin-coverting enzyme. Ultrastructural localization and its recycling from the cell surface. Hypertension. 1998;31:3–9. - PubMed
-
- Boitani C, Palombi F, Stefanini M. Influence of Sertoli cell products upon the in vitro survival of isolated spermatocytes and spermatids. Cell Biol Int Rep. 1983;7:383–393. - PubMed

