Evidence for apical endocytosis in polarized hepatic cells: phosphoinositide 3-kinase inhibitors lead to the lysosomal accumulation of resident apical plasma membrane proteins
- PMID: 10352024
- PMCID: PMC2133136
- DOI: 10.1083/jcb.145.5.1089
Evidence for apical endocytosis in polarized hepatic cells: phosphoinositide 3-kinase inhibitors lead to the lysosomal accumulation of resident apical plasma membrane proteins
Abstract
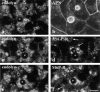
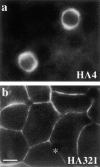
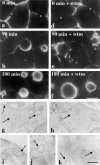
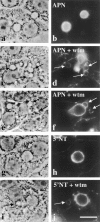
The architectural complexity of the hepatocyte canalicular surface has prevented examination of apical membrane dynamics with methods used for other epithelial cells. By adopting a pharmacological approach, we have documented for the first time the internalization of membrane proteins from the hepatic apical surface. Treatment of hepatocytes or WIF-B cells with phosphoinositide 3-kinase inhibitors, wortmannin or LY294002, led to accumulation of the apical plasma membrane proteins, 5'-nucleotidase and aminopeptidase N in lysosomal vacuoles. By monitoring the trafficking of antibody-labeled molecules, we determined that the apical proteins in vacuoles came from the apical plasma membrane. Neither newly synthesized nor transcytosing apical proteins accumulated in vacuoles. In wortmannin-treated cells, transcytosing apical proteins traversed the subapical compartment (SAC), suggesting that this intermediate in the basolateral-to-apical transcytotic pathway remained functional. Ultrastructural analysis confirmed these results. However, apically internalized proteins did not travel through SAC en route to lysosomal vacuoles, indicating that SAC is not an intermediate in the apical endocytic pathway. Basolateral membrane protein distributions did not change in treated cells, uncovering another difference in endocytosis from the two domains. Similar effects were observed in polarized MDCK cells, suggesting conserved patterns of phosphoinositide 3-kinase regulation among epithelial cells. These results confirm a long-held but unproven assumption that lysosomes are the final destination of apical membrane proteins in hepatocytes. Significantly, they also confirm our hypothesis that SAC is not an apical endosome.
Figures






Similar articles
-
Vps34p differentially regulates endocytosis from the apical and basolateral domains in polarized hepatic cells.J Cell Biol. 2001 Sep 17;154(6):1197-208. doi: 10.1083/jcb.200105138. J Cell Biol. 2001. PMID: 11564757 Free PMC article.
-
Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes.J Cell Biol. 1998 Apr 6;141(1):115-33. doi: 10.1083/jcb.141.1.115. J Cell Biol. 1998. PMID: 9531552 Free PMC article.
-
Wortmannin, an inhibitor of phosphoinositide 3-kinase, inhibits transcytosis in polarized epithelial cells.J Biol Chem. 1995 Nov 24;270(47):28425-32. doi: 10.1074/jbc.270.47.28425. J Biol Chem. 1995. PMID: 7499348
-
Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton.Traffic. 2001 Mar;2(3):149-59. doi: 10.1034/j.1600-0854.2001.020301.x. Traffic. 2001. PMID: 11260520 Review.
-
Mechanisms and functional features of polarized membrane traffic in epithelial and hepatic cells.Biochem J. 1998 Dec 1;336 ( Pt 2)(Pt 2):257-69. doi: 10.1042/bj3360257. Biochem J. 1998. PMID: 9820799 Free PMC article. Review.
Cited by
-
Phosphoinositide 3-kinase regulates the role of retromer in transcytosis of the polymeric immunoglobulin receptor.Exp Cell Res. 2007 Feb 15;313(4):707-18. doi: 10.1016/j.yexcr.2006.11.010. Epub 2006 Nov 23. Exp Cell Res. 2007. PMID: 17184770 Free PMC article.
-
Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains.Mol Biol Cell. 2007 Jul;18(7):2687-97. doi: 10.1091/mbc.e05-09-0873. Epub 2007 May 9. Mol Biol Cell. 2007. PMID: 17494872 Free PMC article.
-
Importance of endocytic pathways in liver function and disease.Compr Physiol. 2014 Oct;4(4):1403-17. doi: 10.1002/cphy.c140001. Compr Physiol. 2014. PMID: 25428849 Free PMC article. Review.
-
A Novel Role of Dickkopf-Related Protein 3 in Macropinocytosis in Human Bladder Cancer T24 Cells.Int J Mol Sci. 2016 Nov 5;17(11):1846. doi: 10.3390/ijms17111846. Int J Mol Sci. 2016. PMID: 27827955 Free PMC article.
-
Membrane Heterogeneity Controls Cellular Endocytic Trafficking.Front Cell Dev Biol. 2020 Aug 5;8:757. doi: 10.3389/fcell.2020.00757. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850860 Free PMC article. Review.
References
-
- Barr VA, Hubbard AL. Newly synthesized hepatocyte plasma membrane proteins are transported in transcytotic vesicles in the bile duct-ligated rat. Gastroenterology. 1993;105:554–571. - PubMed
-
- Barr VA, Scott LJ, Hubbard AL. Immunoadsorption of hepatic vesicles carrying newly synthesized dipeptidyl peptidase IV and polymeric IgA receptor. J Biol Chem. 1995;270:27834–27844. - PubMed
-
- Bartles JR, Braiterman LT, Hubbard AL. Biochemical characterization of domain-specific glycoproteins of the rat hepatocyte plasma membrane. J Biol Chem. 1985;260:12792–12802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

