Auxiliary subunits operate as a molecular switch in determining gating behaviour of the unitary N-type Ca2+ channel current in Xenopus oocytes
- PMID: 10358108
- PMCID: PMC2269381
- DOI: 10.1111/j.1469-7793.1999.0659s.x
Auxiliary subunits operate as a molecular switch in determining gating behaviour of the unitary N-type Ca2+ channel current in Xenopus oocytes
Abstract
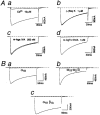
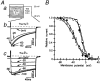
1. We systematically examined the biophysical properties of omega-conotoxin GVIA-sensitive neuronal N-type channels composed of various combinations of the alpha1B, alpha2/delta and beta1b subunits in Xenopus oocytes. 2. Whole-cell recordings demonstrated that coexpression of the beta1b subunit decelerated inactivation, whereas the alpha2/delta accelerated both activation and inactivation, and cancelled the kinetic effects of the beta1b. The alpha2/delta and the beta1b controlled voltage dependence of activation differently: the beta1b significantly shifted the current-voltage relationship towards the hyperpolarizing direction; however, the alpha2/delta shifted the relationship only slightly in the depolarizing direction. The extent of voltage-dependent inactivation was modified solely by the beta1b. 3. Unitary currents measured using a cell-attached patch showed stable patterns of opening that were markedly different among subunit combinations in their kinetic parameters. The alpha2/delta and the beta1b subunits also acted antagonistically in regulating gating patterns of unitary N-type channels. Open time was shortened by the alpha2/delta, while the fraction of long opening was enhanced by the beta1b. The alpha2/delta decreased opening probability (Po), while the beta1b increased Po. alpha1Balpha2/deltabeta1b produced unitary activity with an open time distribution value in between those of alpha1Balpha2/delta and alpha1Bbeta1b. However, both the alpha2/delta and the beta1b subunits reduced the number of null traces. 4. These results suggest that the auxiliary subunits alone and in combination contribute differently in forming gating apparatuses in the N-type channel, raising the possibility that subunit interaction contributes to the generation of functional diversity of N-type channels in native neuronal preparations also.
Figures






Similar articles
-
Subunit regulation of the neuronal alpha 1A Ca2+ channel expressed in Xenopus oocytes.J Physiol. 1995 Jun 15;485 ( Pt 3)(Pt 3):619-34. doi: 10.1113/jphysiol.1995.sp020757. J Physiol. 1995. PMID: 7562605 Free PMC article.
-
Effects of 2,3-butanedione monoxime (BDM) on calcium channels expressed in Xenopus oocytes.J Physiol. 1998 Apr 1;508 ( Pt 1)(Pt 1):1-14. doi: 10.1111/j.1469-7793.1998.001br.x. J Physiol. 1998. PMID: 9490807 Free PMC article.
-
Functional characterization of ion permeation pathway in the N-type Ca2+ channel.J Neurophysiol. 1998 Feb;79(2):622-34. doi: 10.1152/jn.1998.79.2.622. J Neurophysiol. 1998. PMID: 9463426
-
Subunit interaction sites in voltage-dependent Ca2+ channels: role in channel function.Trends Neurosci. 1998 Apr;21(4):148-54. doi: 10.1016/s0166-2236(97)01200-9. Trends Neurosci. 1998. PMID: 9554724 Review.
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
Cited by
-
Functional exofacially tagged N-type calcium channels elucidate the interaction with auxiliary α2δ-1 subunits.Proc Natl Acad Sci U S A. 2014 Jun 17;111(24):8979-84. doi: 10.1073/pnas.1403731111. Epub 2014 Jun 2. Proc Natl Acad Sci U S A. 2014. PMID: 24889613 Free PMC article.
-
The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential.Pharmacol Rev. 2015 Oct;67(4):821-70. doi: 10.1124/pr.114.009654. Pharmacol Rev. 2015. PMID: 26362469 Free PMC article. Review.
-
Proteolytic maturation of α2δ represents a checkpoint for activation and neuronal trafficking of latent calcium channels.Elife. 2016 Oct 26;5:e21143. doi: 10.7554/eLife.21143. Elife. 2016. PMID: 27782881 Free PMC article.
-
Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology.J Physiol. 2016 Oct 1;594(19):5369-90. doi: 10.1113/JP272262. Epub 2016 Jul 5. J Physiol. 2016. PMID: 27273705 Free PMC article. Review.
-
Interaction via a key tryptophan in the I-II linker of N-type calcium channels is required for beta1 but not for palmitoylated beta2, implicating an additional binding site in the regulation of channel voltage-dependent properties.J Neurosci. 2005 Jul 27;25(30):6984-96. doi: 10.1523/JNEUROSCI.1137-05.2005. J Neurosci. 2005. PMID: 16049174 Free PMC article.
References
-
- Ahlijanian MK, Westenbroek RE, Catterall WA. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord and retina. Neuron. 1990;4:819–832. 10.1016/0896-6273(90)90135-3. - DOI - PubMed
-
- Anwyl R. Modulation of vertebrate neuronal calcium channels by transmitters. Brain Research Reviews. 1991;16:265–281. 10.1016/0165-0173(91)90010-6. - DOI - PubMed
-
- Aosaki T, Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and ω-conotoxin GVIA. Pflügers Archiv. 1989;414:150–156. - PubMed
-
- Bean BP. Classes of calcium channels in vertebrate cells. Annual Review of Physiology. 1989;51:367–384. - PubMed
-
- Brust PF, Simerson S, McCue AF, Deal CR, Schoonmaker S, Williams ME, Velicelebi G, Johnson EC, Harpold MM, Ellis SB. Human neuronal voltage-dependent calcium channels: studies on subunit structure and role in channel assembly. Neuropharmacology. 1993;32:1089–1102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

