Effects of galvanic vestibular stimulation during human walking
- PMID: 10358131
- PMCID: PMC2269389
- DOI: 10.1111/j.1469-7793.1999.0931s.x
Effects of galvanic vestibular stimulation during human walking
Abstract
1. To identify vestibular influences on human walking, galvanic vestibular stimulation was applied to normal adult subjects as they walked to a previously seen target. A transmastoidal step stimulus commenced as subjects started walking. With the eyes shut, the galvanic stimulus caused large turns towards the side with the anodal current. 2. Ability to perceive the trajectory of gait without visual cues was measured by guiding blindfolded subjects from one arbitrary point to another, either walking or seated in a wheelchair. On reaching a destination position and removing the blindfold, subjects pointed to indicate the starting position. Subjects made considerable errors in estimating the trajectory, but were equally accurate whether in the wheelchair or walking. 3. To determine the effects of vestibular stimulation on the perception of trajectory, the galvanic stimulus was applied to blindfolded subjects as they were guided from one point to another in the wheelchair. The vestibular stimulus produced an illusory shift in the trajectory travelled. This shift was towards the side with the cathode, i.e. in the opposite direction to the turn produced by the stimulus during walking. 4. We conclude that galvanic vestibular stimulation during walking causes subjects to turn from their planned trajectory. In part, this altered course may compensate for an altered perception of trajectory produced by the stimulus. However, altered perception of the vertical or the base of support, or direct vestibulo-fugal influences on the leg muscles could contribute to the changes in gait.
Figures
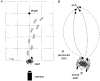
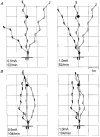

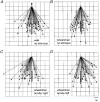
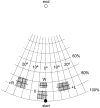
Comment in
-
Galvanic vestibular stimulation: new uses for an old tool.J Physiol. 1999 Jun 15;517 ( Pt 3)(Pt 3):631. doi: 10.1111/j.1469-7793.1999.0631s.x. J Physiol. 1999. PMID: 10358104 Free PMC article. No abstract available.
References
-
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Experimental Brain Research. 1993;94:143–151. - PubMed
-
- Cass SP, Redfern MS, Furman JM, DiPasquale JJ. Galvanic-induced postural movements as a test of vestibular function in humans. Laryngoscope. 1996;106:423–430. - PubMed
-
- Coates AC. Effect of varying stimulus parameters on the galvanic body-sway response. Annals of Otology, Rhinology and Laryngology. 1973;82:96–102. - PubMed
-
- Courjon JH, Precht W, Sirkin DW. Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic stimulation of the labyrinth in the rat. Experimental Brain Research. 1987;66:41–48. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

