Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting
- PMID: 10359558
- PMCID: PMC408372
- DOI: 10.1172/JCI6223
Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting
Abstract
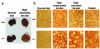
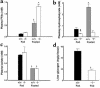
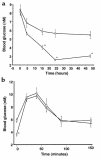
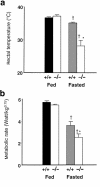
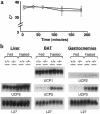
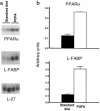
Prolonged deprivation of food induces dramatic changes in mammalian metabolism, including the release of large amounts of fatty acids from the adipose tissue, followed by their oxidation in the liver. The nuclear receptor known as peroxisome proliferator-activated receptor alpha (PPARalpha) was found to play a role in regulating mitochondrial and peroxisomal fatty acid oxidation, suggesting that PPARalpha may be involved in the transcriptional response to fasting. To investigate this possibility, PPARalpha-null mice were subjected to a high fat diet or to fasting, and their responses were compared with those of wild-type mice. PPARalpha-null mice chronically fed a high fat diet showed a massive accumulation of lipid in their livers. A similar phenotype was noted in PPARalpha-null mice fasted for 24 hours, who also displayed severe hypoglycemia, hypoketonemia, hypothermia, and elevated plasma free fatty acid levels, indicating a dramatic inhibition of fatty acid uptake and oxidation. It is shown that to accommodate the increased requirement for hepatic fatty acid oxidation, PPARalpha mRNA is induced during fasting in wild-type mice. The data indicate that PPARalpha plays a pivotal role in the management of energy stores during fasting. By modulating gene expression, PPARalpha stimulates hepatic fatty acid oxidation to supply substrates that can be metabolized by other tissues.
Figures

References
-
- van den Berghe G. The role of the liver in metabolic homeostasis: implications for inborn errors of metabolism. J Inherit Metab Dis. 1991;14:407–420. - PubMed
-
- Desvergne B, IJpenberg A, Devchand PR, Wahli W. The peroxisome proliferator-activated receptors at the cross-road of diet and hormonal signalling. J Steroid Biochem Mol Biol. 1998;65:65–74. - PubMed
-
- Gronemeyer H, Laudet V. Transcription factors 3: nuclear receptors. Protein Profile. 1995;2:1173–1308. - PubMed
-
- Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37:907–925. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

