Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension
- PMID: 10359560
- PMCID: PMC408370
- DOI: 10.1172/JCI5911
Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension
Abstract
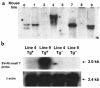
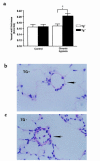
Prostacyclin synthase (PGIS) is the final committed enzyme in the metabolic pathway leading to prostacyclin (PGI2) production. Patients with severe pulmonary hypertension have a PGIS deficiency of their precapillary vessels, but the importance of this deficiency for lung vascular remodeling remains unclear. We hypothesized that selective pulmonary overexpression of PGIS may prevent the development of pulmonary hypertension. To study this hypothesis, transgenic mice were created with selective pulmonary PGIS overexpression using a construct of the 3.7-kb human surfactant protein-C (SP-C) promoter and the rat PGIS cDNA. Transgenic mice (Tg+) and nontransgenic littermates (Tg-) were subjected to a simulated altitude of 17,000 ft for 5 weeks, and right ventricular systolic pressure (RVSP) was measured. Histology was performed on the lungs. The Tg+ mice produced 2-fold more pulmonary 6-keto prostaglandin F1alpha (PGF1alpha) levels than did Tg- mice. After exposure to chronic hypobaric hypoxia, Tg+ mice have lower RVSP than do Tg- mice. Histologic examination of the lungs revealed nearly normal arteriolar vessels in the Tg+ mice in comparison with vessel wall hypertrophy in the Tg- mice. These studies demonstrate that Tg+ mice were protected from the development of pulmonary hypertension after exposure to chronic hypobaric hypoxia. We conclude that PGIS plays a major role in modifying the pulmonary vascular response to chronic hypoxia. This has important implications for the pathogenesis and treatment of severe pulmonary hypertension.
Figures
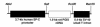




References
-
- Bunting S, Gryglewski R, Moncada S, Vane JR. Arterial walls generate from prostaglandin endoperoxidases a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac arteries and inhibits platelet aggregation. Prostaglandins. 1976;12:897–913. - PubMed
-
- Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1979;30:293–331. - PubMed
-
- Owen, N.E. 1985. Prostacyclin can inhibit DNA synthesis in vascular smooth muscle cells. In Prostaglandins, leukotrienes, and lipoxins. J.M. Bailey, editor. Plenum Press. New York, NY. 193–204.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

