Mild spherocytosis and altered red cell ion transport in protein 4. 2-null mice
- PMID: 10359562
- PMCID: PMC408368
- DOI: 10.1172/JCI5766
Mild spherocytosis and altered red cell ion transport in protein 4. 2-null mice
Abstract
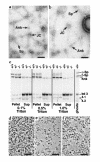
Protein 4.2 is a major component of the red blood cell (RBC) membrane skeleton. We used targeted mutagenesis in embryonic stem (ES) cells to elucidate protein 4.2 functions in vivo. Protein 4. 2-null (4.2(-/-)) mice have mild hereditary spherocytosis (HS). Scanning electron microscopy and ektacytometry confirm loss of membrane surface in 4.2(-/-) RBCs. The membrane skeleton architecture is intact, and the spectrin and ankyrin content of 4. 2(-/-) RBCs are normal. Band 3 and band 3-mediated anion transport are decreased. Protein 4.2(-/-) RBCs show altered cation content (increased K+/decreased Na+)resulting in dehydration. The passive Na+ permeability and the activities of the Na-K-2Cl and K-Cl cotransporters, the Na/H exchanger, and the Gardos channel in 4. 2(-/-) RBCs are significantly increased. Protein 4.2(-/-) RBCs demonstrate an abnormal regulation of cation transport by cell volume. Cell shrinkage induces a greater activation of Na/H exchange and Na-K-2Cl cotransport in 4.2(-/-) RBCs compared with controls. The increased passive Na+ permeability of 4.2(-/-) RBCs is also dependent on cell shrinkage. We conclude that protein 4.2 is important in the maintenance of normal surface area in RBCs and for normal RBC cation transport.
Figures


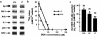
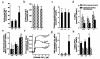
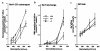
References
-
- Korsgren C, Cohen CM. cDNA sequence, gene structure, and properties of murine pallidin (band 4.2), the protein implicated in the murine pallid mutation. Genomics. 1994;21:478–485. - PubMed
-
- Lux, S.E., and Palek, J. 1995. Disorders of the red cell membrane. In Blood: principles and practice of hematology. R.I. Handin, S.E. Lux, and T.P. Stossel, editors. J.B. Lippincott. Philadelphia, PA. 1701–1818.
-
- Yawata Y. Red cell membrane protein band 4.2: phenotypic, genetic and electron microscopic aspects. Biochim Biophys Acta. 1994;1204:131–148. - PubMed
-
- Korsgren C, Cohen CM. Associations of human erythrocyte protein 4.2. Binding to ankyrin and to the cytoplasmic domain of band 3. J Biol Chem. 1988;263:10212–10218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

