Secondary rearrangements and hypermutation generate sufficient B cell diversity to mount protective antiviral immunoglobulin responses
- PMID: 10359583
- PMCID: PMC2193076
- DOI: 10.1084/jem.189.11.1791
Secondary rearrangements and hypermutation generate sufficient B cell diversity to mount protective antiviral immunoglobulin responses
Abstract
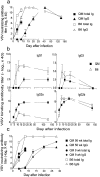
Variable (V) region gene replacement was recently implicated in B cell repertoire diversification, but the contribution of this mechanism to antibody responses is still unknown. To investigate the role of V gene replacements in the generation of antigen-specific antibodies, we analyzed antiviral immunoglobulin responses of "quasimonoclonal" (QM) mice. The B cells of QM mice are genetically committed to exclusively express the anti-(4-hydroxy-3-nitrophenyl) acetyl specificity. However, approximately 20% of the peripheral B cells of QM mice undergo secondary rearrangements and thereby potentially acquire new specificities. QM mice infected with vesicular stomatitis virus (VSV), lymphocytic choriomeningitis virus, or poliovirus mounted virus-specific neutralizing antibody responses. In general, kinetics of the antiviral immunoglobulin responses were delayed in QM mice; however, titers similar to control animals were eventually produced that were sufficient to protect against VSV-induced lethal disease. VSV neutralizing single-chain Fv fragments isolated from phage display libraries constructed from QM mice showed VH gene replacements and extensive hypermutation. Thus, our data demonstrate that secondary rearrangements and hypermutation can generate sufficient B cell diversity in QM mice to mount protective antiviral antibody responses, suggesting that these mechanisms might also contribute to the diversification of the B cell repertoire of normal mice.
Figures