The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated integrin suppression pathway
- PMID: 10359597
- PMCID: PMC25373
- DOI: 10.1091/mbc.10.6.1799
The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated integrin suppression pathway
Abstract
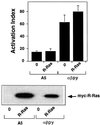
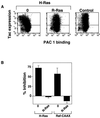
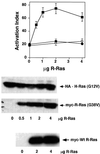
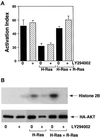
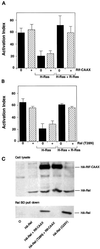
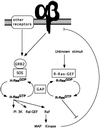
The rapid modulation of ligand-binding affinity ("activation") is a central property of the integrin family of cell adhesion receptors. The small GTP-binding protein Ras and its downstream effector kinase Raf-1 suppress integrin activation. In this study we explored the relationship between Ras and the closely related small GTP-binding protein R-Ras in modulating the integrin affinity state. We found that R-Ras does not seem to be a direct activator of integrins in Chinese hamster ovary cells. However, we observed that GTP-bound R-Ras strongly antagonizes the Ras/Raf-initiated integrin suppression pathway. Furthermore, this reversal of the Ras/Raf suppressor pathway does not seem to be via a competition between Ras and R-Ras for common downstream effectors or via an inhibition of Ras/Raf-induced MAP kinase activation. Thus, R-Ras and Ras may act in concert to regulate integrin affinity via the activation of distinct downstream effectors.
Figures

References
-
- Bos JL. Ras-like GTPases. Biochim Biophys Acta. 1997;1333:19–31. - PubMed
-
- Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–148. - PubMed
-
- Cox AD, Brtva TR, Lowe DG, Der CJ. R-Ras induces malignant, but not morphologic, transformation of NIH3T3 cells. Oncogene. 1994;9:3281–3288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

