Erp1p and Erp2p, partners for Emp24p and Erv25p in a yeast p24 complex
- PMID: 10359606
- PMCID: PMC25390
- DOI: 10.1091/mbc.10.6.1923
Erp1p and Erp2p, partners for Emp24p and Erv25p in a yeast p24 complex
Abstract
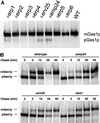
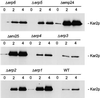
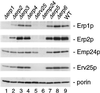
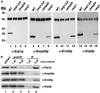
Six new members of the yeast p24 family have been identified and characterized. These six genes, named ERP1-ERP6 (for Emp24p- and Erv25p-related proteins) are not essential, but deletion of ERP1 or ERP2 causes defects in the transport of Gas1p, in the retention of BiP, and deletion of ERP1 results in the suppression of a temperature-sensitive mutation in SEC13 encoding a COPII vesicle coat protein. These phenotypes are similar to those caused by deletion of EMP24 or ERV25, two previously identified genes that encode related p24 proteins. Genetic and biochemical studies demonstrate that Erp1p and Erp2p function in a heteromeric complex with Emp24p and Erv25p.
Figures





References
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed
-
- Bednarek SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. - PubMed
-
- Belden WJ, Barlowe C. Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:26939–26946. - PubMed
-
- Blum R, Feick P, Puype M, Vandekerckhove J, Klengel R, Nastainczyk W, Schulz I. Tmp21 and p24a, two type I proteins enriched in pancreatic microsomal membranes, are members of a protein family involved in vesicular trafficking. J Biol Chem. 1996;271:17183–17189. - PubMed
-
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

