Congruent docking of dimeric kinesin and ncd into three-dimensional electron cryomicroscopy maps of microtubule-motor ADP complexes
- PMID: 10359615
- PMCID: PMC25414
- DOI: 10.1091/mbc.10.6.2063
Congruent docking of dimeric kinesin and ncd into three-dimensional electron cryomicroscopy maps of microtubule-motor ADP complexes
Abstract
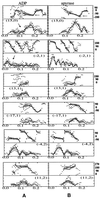
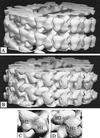
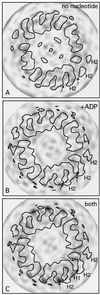
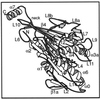
We present a new map showing dimeric kinesin bound to microtubules in the presence of ADP that was obtained by electron cryomicroscopy and image reconstruction. The directly bound monomer (first head) shows a different conformation from one in the more tightly bound empty state. This change in the first head is amplified as a movement of the second (tethered) head, which tilts upward. The atomic coordinates of kinesin.ADP dock into our map so that the tethered head associates with the bound head as in the kinesin dimer structure seen by x-ray crystallography. The new docking orientation avoids problems associated with previous predictions; it puts residues implicated by proteolysis-protection and mutagenesis studies near the microtubule but does not lead to steric interference between the coiled-coil tail and the microtubule surface. The observed conformational changes in the tightly bound states would probably bring some important residues closer to tubulin. As expected from the homology with kinesin, the atomic coordinates of nonclaret disjunctional protein (ncd).ADP dock in the same orientation into the attached head in a map of microtubules decorated with dimeric ncd.ADP. Our results support the idea that the observed direct interaction between the two heads is important at some stages of the mechanism by which kinesin moves processively along microtubules.
Figures




Similar articles
-
A new look at the microtubule binding patterns of dimeric kinesins.J Mol Biol. 2000 Apr 14;297(5):1087-103. doi: 10.1006/jmbi.2000.3627. J Mol Biol. 2000. PMID: 10764575
-
3D electron microscopy of the interaction of kinesin with tubulin.Cell Struct Funct. 1999 Oct;24(5):277-84. doi: 10.1247/csf.24.277. Cell Struct Funct. 1999. PMID: 15216883
-
Nucleotide-dependent structural changes in dimeric NCD molecules complexed to microtubules.J Mol Biol. 1998 May 1;278(2):389-400. doi: 10.1006/jmbi.1998.1709. J Mol Biol. 1998. PMID: 9571059
-
Kinesins and microtubules: their structures and motor mechanisms.Curr Opin Cell Biol. 2000 Feb;12(1):35-41. doi: 10.1016/s0955-0674(99)00054-x. Curr Opin Cell Biol. 2000. PMID: 10679355 Review.
-
Kinesin, 30 years later: Recent insights from structural studies.Protein Sci. 2015 Jul;24(7):1047-56. doi: 10.1002/pro.2697. Epub 2015 Jun 11. Protein Sci. 2015. PMID: 25975756 Free PMC article. Review.
Cited by
-
Nucleotide-induced conformations in the neck region of dimeric kinesin.EMBO J. 2003 Apr 1;22(7):1518-28. doi: 10.1093/emboj/cdg164. EMBO J. 2003. PMID: 12660159 Free PMC article.
-
An ATP gate controls tubulin binding by the tethered head of kinesin-1.Science. 2007 Apr 6;316(5821):120-3. doi: 10.1126/science.1136985. Science. 2007. PMID: 17412962 Free PMC article.
-
Kinesin's processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains.Proc Natl Acad Sci U S A. 1999 Nov 9;96(23):13147-52. doi: 10.1073/pnas.96.23.13147. Proc Natl Acad Sci U S A. 1999. PMID: 10557288 Free PMC article.
-
Kinesin processivity.J Cell Biol. 2000 Nov 27;151(5):F27-9. doi: 10.1083/jcb.151.5.f27. J Cell Biol. 2000. PMID: 11086015 Free PMC article. Review. No abstract available.
-
The conformational cycle of kinesin.Philos Trans R Soc Lond B Biol Sci. 2000 Apr 29;355(1396):459-64. doi: 10.1098/rstb.2000.0587. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10836499 Free PMC article. Review.
References
-
- Amos LA, Hirose K. The structure of microtubule-motor complexes. Curr Opin Cell Biol. 1997;9:4–11. - PubMed
-
- Arnal I, Metoz F, DeBonis S, Wade RH. Three-dimensional structure of functional motor proteins on microtubules. Curr Biol. 1996;6:1265–1270. - PubMed
-
- Arnal I, Wade RH. Nucleotide-dependent conformations of the kinesin dimer interacting with microtubules. Structure. 1998;6:33–38. - PubMed
-
- Crevel IM-T, Lockhart A, Cross RA. Weak and strong states of kinesin and ncd. J Mol Biol. 1996;257:66–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

