Hemoglobin induction in mouse macrophages
- PMID: 10359765
- PMCID: PMC21968
- DOI: 10.1073/pnas.96.12.6643
Hemoglobin induction in mouse macrophages
Abstract
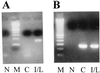
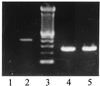
The common perception that hemoglobin is involved solely in the transport of oxygen and carbon dioxide has been challenged by recent studies with nitric oxide (NO). These studies have shown that the primordial bacterial flavohemoglobin functions to consume NO enzymatically (to protect from nitrosative stress), whereas mammalian hemoglobin functions to deliver NO (thus maximizing oxygen delivery in the respiratory cycle). Here we report that murine macrophages stimulated to produce NO with lipopolysaccharide and interferon-gamma express the betaminor hemoglobin subunit. Consumption of NO, however, was not increased by cytokines or by hemoglobin expression. These data suggest alternative functions for globins in mammalian cells, and they challenge the prevailing view that the expression of alpha- and beta-globin genes is always balanced and coordinated.
Figures




References
-
- Perutz M F. In: Molecular Basis of Blood Diseases. Stammatayanopoulos G, editor. Philadelphia: Saunders; 1987. pp. 127–178.
-
- Poole R K. Antonie Leeuwenhoek. 1994;65:289–310. - PubMed
-
- Jia L, Bonaventura C, Bonaventura J, Stamler J S. Nature (London) 1996;380:221–226. - PubMed
-
- Stamler J S, Jia L, Eu J P, McMahon T J, Demchenko I T, Bonaventura J, Gernert K, Piantadosi C A. Science. 1997;276:2034–2037. - PubMed
-
- Gow A J, Stamler J S. Nature (London) 1998;391:169–173. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

