Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation
- PMID: 10359785
- PMCID: PMC21988
- DOI: 10.1073/pnas.96.12.6757
Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation
Abstract
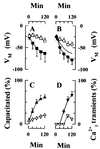
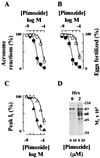
Sperm adhesion to egg zonae pellucidae initiates sperm acrosome reactions, an exocytotic event that is an early step during fertilization. Previously, it was suggested that zona pellucida-evoked Ca2+ entry into sperm through low voltage-activated Ca2+ channels is an essential step in acrosome reactions, based on the inhibitory effects of Ca2+ channel antagonists. However, analysis of this channel is limited by the inability to apply electrophysiological methods directly to sperm. In this report, optical methods of determining membrane potential and internal Ca2+ levels were used to demonstrate that (i) contact with zonae pellucidae activates a transient Ca2+ response in sperm that has a time course and antagonist sensitivity anticipated of low voltage-activated Ca2+ channels; (ii) these channels are unavailable for opening in uncapacitated sperm because of voltage-dependent, steady state inactivation; (iii) membrane hyperpolarization during sperm capacitation is sufficient to recruit channels into a closed state, from which they are available for opening during fertilization; and (iv) channel conductance state may be a factor in determines the efficacy with which channel antagonists inhibit fertilization. This study provides evidence for the activation of sperm Ca2+ channels during gamete adhesion and offers a mechanism that may account for aspects of the regulation of sperm fertility during capacitation through the control of channel availability. Finally, these results suggest that channel conductance state may be a central feature in the design of channel antagonists that inhibit sperm function.
Figures
References
-
- Yanagimachi R. In: Mammalian Fertilization. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 189–317.
-
- Wassarman P M, Florman H M. In: Handbook of Physiology. Hoffman J F, Jamieson J, editors. Vol. 14. New York: Oxford Univ Press; 1997. pp. 885–938.
-
- Wassarman P M. Cell. 1999;95:176–183.
-
- Benoff S, Cooper G W, Hurley I, Mandel F S, Rosenfeld D L, Scholl G M, Gilbert B R, Hershlag A. Fertil Steril. 1994;62:606–617. - PubMed
-
- Hershlag A, Cooper G W, Benoff S. Hum Reprod. 1995;10:599–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

