Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB
- PMID: 10359807
- PMCID: PMC22010
- DOI: 10.1073/pnas.96.12.6879
Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB
Abstract
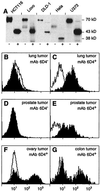
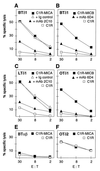
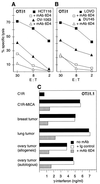
Human MHC class I-related molecules, MICA and MICB, are stress-induced antigens that are recognized by a subset of gamma delta T cells expressing the variable region Vdelta1. This functional association has been found to be limited to intestinal epithelium, where these T cells are prevalent and where MICA and, presumably, MICB are mainly expressed. However, increased frequencies of Vdelta1 gamma delta T cells have been observed in various epithelial tumors; moreover, MICA/B are expressed on diverse cultured epithelial tumor cells. With freshly isolated tumor specimens, expression of MICA/B was documented in many, but not all, carcinomas of the lung, breast, kidney, ovary, prostate, and colon. In tumors that were positive for MICA/B, the frequencies of Vdelta1 gamma delta T cells were significantly higher than in those that were negative. Vdelta1 gamma delta T cell lines and clones derived from different tumors recognized MICA/B on autologous and heterologous tumor cells. In accord with previous evidence, no constraints were observed in these interactions, such as those imposed by specific peptide ligands. Thus, MICA/B are tumor-associated antigens that can be recognized, in an apparently unconditional manner, by a subset of tumor-infiltrating gamma delta T cells. These results raise the possibility that an induced expression of MICA/B, by conditions that may be related to tumor homeostasis and growth, could play a role in immune responses against tumors.
Figures

References
-
- Van den Eynde B J, van der Bruggen P. Curr Opin Immunol. 1997;9:684–693. - PubMed
-
- Pamer E, Cresswell P. Annu Rev Immunol. 1998;16:323–358. - PubMed
-
- Rammensee H-G, Falk K, Ràtzschke O. Annu Rev Immunol. 1993;11:213–244. - PubMed
-
- Ferrone S, Marincola F M. Immunol Today. 1995;16:487–494. - PubMed
-
- Allison J P, Raulet D H. Semin Immunol. 1990;2:59–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

