Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment
- PMID: 10359810
- PMCID: PMC22013
- DOI: 10.1073/pnas.96.12.6896
Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment
Abstract
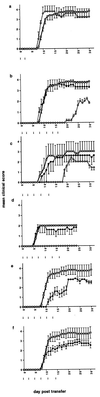
The role of various adhesion molecules in lymphocyte homing to the brain and in inflammatory autoimmune disease of the central nervous system (CNS) was examined in mice. Activated T cell lines and clones expressed CD44 and integrin alpha4, but not L-selectin, and entered the CNS independent of their antigen specificity. mAbs directed against CD44 and integrin alpha4 prevented the transfer of experimental autoimmune encephalomyelitis (EAE) by myelin basic protein-specific T cells. T cells preincubated with anti-CD44 or antiintegrin alpha4 were blocked only partially from entering the brain parenchyma. However, both antibodies efficiently prevented CNS inflammation and clinical expression of EAE when injected in vivo. This effect lasted as long as antibodies were administered. Antibodies specific for L-selectin had no effect on homing of encephalitogenic T cells to the brain or development of EAE. Antiintegrin alpha4 and anti-CD44 did not impair the activation and function of encephalitogenic T cells in vitro and did not deplete integrin alpha4- or CD44-positive cells in vivo. These data suggest that, in the absence of leukocyte recruitment, the entry of a reduced number of activated myelin basic protein-reactive T cells in the CNS is not sufficient for the development and expression of EAE. We propose that antibodies to integrin alpha4 and CD44 prevent clinical disease by partially targeting the primary influx of encephalitogenic T cells and by preventing the secondary influx of leukocytes to lesions initiated by the transferred T cells.
Figures


Similar articles
-
Overlapping roles of P-selectin and alpha 4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis.J Immunol. 2002 Jul 15;169(2):1000-6. doi: 10.4049/jimmunol.169.2.1000. J Immunol. 2002. PMID: 12097407
-
Beneficial effect of modified peptide inhibitor of alpha4 integrins on experimental allergic encephalomyelitis in Lewis rats.J Neurosci Res. 2002 Jan 15;67(2):191-9. doi: 10.1002/jnr.10095. J Neurosci Res. 2002. PMID: 11782963
-
The development of experimental autoimmune encephalomyelitis in the mouse requires alpha4-integrin but not alpha4beta7-integrin.J Clin Invest. 1998 Dec 15;102(12):2096-105. doi: 10.1172/JCI4271. J Clin Invest. 1998. PMID: 9854045 Free PMC article.
-
Immune cell entry into the central nervous system: involvement of adhesion molecules and chemokines.J Neurol Sci. 2008 Nov 15;274(1-2):23-6. doi: 10.1016/j.jns.2008.05.019. Epub 2008 Jun 24. J Neurol Sci. 2008. PMID: 18573502 Review.
-
Lymphocyte targeting of the central nervous system: a review of afferent and efferent CNS-immune pathways.Brain Pathol. 1996 Jul;6(3):275-88. doi: 10.1111/j.1750-3639.1996.tb00855.x. Brain Pathol. 1996. PMID: 8864284 Review.
Cited by
-
Co-transplantation of pure blood stem cells with antigen-specific but not bulk T cells augments functional immunity.Proc Natl Acad Sci U S A. 2012 Apr 10;109(15):5820-5. doi: 10.1073/pnas.1120237109. Epub 2012 Mar 22. Proc Natl Acad Sci U S A. 2012. PMID: 22440752 Free PMC article.
-
Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells.Neurology. 2009 Jun 2;72(22):1922-30. doi: 10.1212/WNL.0b013e3181a8266f. Neurology. 2009. PMID: 19487650 Free PMC article.
-
Mechanism of action and efficacy of RX-111, a thieno[2,3-c]pyridine derivative and small molecule inhibitor of protein interaction with glycosaminoglycans (SMIGs), in delayed-type hypersensitivity, TNBS-induced colitis and experimental autoimmune encephalomyelitis.Inflamm Res. 2016 Apr;65(4):285-94. doi: 10.1007/s00011-016-0915-4. Epub 2016 Jan 21. Inflamm Res. 2016. PMID: 26794621
-
Therapy with recombinant T-cell receptor ligand reduces infarct size and infiltrating inflammatory cells in brain after middle cerebral artery occlusion in mice.Metab Brain Dis. 2011 Jun;26(2):123-33. doi: 10.1007/s11011-011-9241-2. Epub 2011 Apr 7. Metab Brain Dis. 2011. PMID: 21472429 Free PMC article.
-
Hyaluronan anchored to activated CD44 on central nervous system vascular endothelial cells promotes lymphocyte extravasation in experimental autoimmune encephalomyelitis.J Biol Chem. 2012 Sep 28;287(40):33237-51. doi: 10.1074/jbc.M112.356287. Epub 2012 Aug 3. J Biol Chem. 2012. PMID: 22865853 Free PMC article.
References
-
- Weissman I L. Cell. 1994;76:207–218. - PubMed
-
- Butcher E C, Picker L J. Science. 1996;272:60–66. - PubMed
-
- Ford W L, Gowans J L. Semin Hematol. 1969;6:67–83. - PubMed
-
- Mackay C. Curr Opin Immunol. 1993;5:423–427. - PubMed
-
- Dailey M O, Fathman C G, Butcher E C, Pillemer E, Weissman I L. J Immunol. 1982;128:2134–2136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

