Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice
- PMID: 10359814
- PMCID: PMC22017
- DOI: 10.1073/pnas.96.12.6920
Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice
Abstract
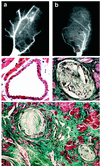
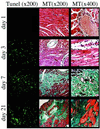
Myocardial infarction is linked to atherosclerosis, yet the sequence leading from silent coronary atherosclerosis to acute myocardial infarction has remained unclear. Here we show that hypercholesterolemic apolipoprotein E-/- low density lipoprotein receptor-/- mice develop not only coronary atherosclerosis but also myocardial infarction. Exposure of mice to mental stress or hypoxia led to acute ischemia, which, in a large proportion of the mice, was followed by electrocardiographic changes, leakage of troponin T, and loss of dehydrogenase from the myocardium, all indicative of acute myocardial infarction. Apoptotic death of cardiomyocytes was followed by inflammation and fibrosis in the heart. All these pathological changes could be prevented by a blocker of the endothelin type A receptor. Thus, stress elicits myocardial infarction through endothelin receptor signaling in coronary atherosclerosis caused by hypercholesterolemia.
Figures


References
-
- Murray C J, Lopez A D. Lancet. 1997;349:1436–1442. - PubMed
-
- Ross R. N Engl J Med. 1999;340:115–126. - PubMed
-
- Libby P. Circulation. 1995;91:2844–2850. - PubMed
-
- Breslow J L. Science. 1996;272:685–688. - PubMed
-
- Plump A S, Smith J D, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft J G, Rubin E M, Breslow J L. Cell. 1992;71:343–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

