Calexcitin transformation of GABAergic synapses: from excitation filter to amplifier
- PMID: 10359832
- PMCID: PMC22042
- DOI: 10.1073/pnas.96.12.7023
Calexcitin transformation of GABAergic synapses: from excitation filter to amplifier
Abstract
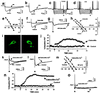
Encoding an experience into a lasting memory is thought to involve an altered operation of relevant synapses and a variety of other subcellular processes, including changed activity of specific proteins. Here, we report direct evidence that co-applying (associating) membrane depolarization of rat hippocampal CA1 pyramidal cells with intracellular microinjections of calexcitin (CE), a memory-related signaling protein, induces a long-term transformation of inhibitory postsynaptic potentials from basket interneurons (BAS) into excitatory postsynaptic potentials. This synaptic transformation changes the function of the synaptic inputs from excitation filter to amplifier, is accompanied by a shift of the reversal potential of BAS-CA1 postsynaptic potentials, and is blocked by inhibiting carbonic anhydrase or antagonizing ryanodine receptors. Effects in the opposite direction are produced when anti-CE antibody is introduced into the cells, whereas heat-inactivated CE and antibodies are ineffective. These data suggest that CE is actively involved in shaping BAS-CA1 synaptic plasticity and controlling information processing through the hippocampal networks.
Figures
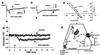

References
-
- Kornhauser J M, Greenberg M E. Neuron. 1997;18:839–842. - PubMed
-
- Alkon D L, Nelson T J, Zhao W Q, Cavallaro S. Trends Neurosci. 1998;21:529–537. - PubMed
-
- Nelson T J, Collin C, Alkon D L. Science. 1990;247:1479–1483. - PubMed
-
- Sun M-K. Pharmacol Rev. 1996;48:465–494. - PubMed
-
- McMahon L L, Kauer J A. Neuron. 1997;18:295–305. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

