Guanosine triphosphatase stimulation of oncogenic Ras mutants
- PMID: 10359839
- PMCID: PMC22057
- DOI: 10.1073/pnas.96.12.7065
Guanosine triphosphatase stimulation of oncogenic Ras mutants
Abstract
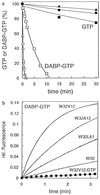
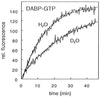
Interest in the guanosine triphosphatase (GTPase) reaction of Ras as a molecular drug target stems from the observation that, in a large number of human tumors, Ras is characteristically mutated at codons 12 or 61, more rarely 13. Impaired GTPase activity, even in the presence of GTPase activating proteins, has been found to be the biochemical reason behind the oncogenicity of most Gly12/Gln61 mutations, thus preventing Ras from being switched off. Therefore, these oncogenic Ras mutants remain constitutively activated and contribute to the neoplastic phenotype of tumor cells. Here, we show that the guanosine 5'-triphosphate (GTP) analogue diaminobenzophenone-phosphoroamidate-GTP (DABP-GTP) is hydrolyzed by wild-type Ras but more efficiently by frequently occurring oncogenic Ras mutants, to yield guanosine 5'-diphosphate-bound inactive Ras and DABP-Pi. The reaction is independent of the presence of Gln61 and is most dramatically enhanced with Gly12 mutants. Thus, the defective GTPase reaction of the oncogenic Ras mutants can be rescued by using DABP-GTP instead of GTP, arguing that the GTPase switch of Ras is not irreversibly damaged. An exocyclic aromatic amino group of DABP-GTP is critical for the reaction and bypasses the putative rate-limiting step of the intrinsic Ras GTPase reaction. The crystal structures of Ras-bound DABP-beta,gamma-imido-GTP show a disordered switch I and identify the Gly12/Gly13 region as the hydrophobic patch to accommodate the DABP-moiety. The biochemical and structural studies help to define the requirements for the design of anti-Ras drugs aimed at the blocked GTPase reaction.
Figures



References
-
- Nicolson G L. Cancer Res. 1987;47:1473–1487. - PubMed
-
- Kinzler K W, Vogelstein B. Cell. 1996;87:159–170. - PubMed
-
- Bourne H R, Sanders D A, McCormick F. Nature (London) 1990;348:125–132. - PubMed
-
- Bourne H R, Sanders D A, McCormick F. Nature (London) 1991;349:117–127. - PubMed
-
- Boguski M S, McCormick F. Nature (London) 1993;366:643–654. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

