Porins and lipopolysaccharide (LPS) from Salmonella typhimurium induce leucocyte transmigration through human endothelial cells in vitro
- PMID: 10361234
- PMCID: PMC1905308
- DOI: 10.1046/j.1365-2249.1999.00904.x
Porins and lipopolysaccharide (LPS) from Salmonella typhimurium induce leucocyte transmigration through human endothelial cells in vitro
Expression of concern in
-
Expression of Concern: Porins and lipopolysaccharide (LPS) from Salmonella typhimurium induce leucocyte transmigration through human endothelial cells in vitro.Clin Exp Immunol. 2025 Jan 21;219(1):uxaf021. doi: 10.1093/cei/uxaf021. Clin Exp Immunol. 2025. PMID: 40338040 Free PMC article. No abstract available.
Abstract
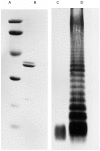
Bacteria or bacterial products may constitute important inducers of surface molecule expression on endothelial cells and leucocytes. This study was undertaken to determine the effects of the Salmonella typhimurium porins, LPS-S and LPS-R on the transendothelial migration of leucocytes through human umbilical vein endothelial cells (HUVEC). Treatment of the HUVEC with either porins or LPS-S or LPS-R increased the transmigration of different leucocyte populations, in particular that of neutrophils. The maximal increase occurred using LPS-S treatment, whereas porin stimulation fell between LPS-S and LPS-R. The transmigration increase was dose-dependent and reached its maximum at about 100-1000 ng/ml of stimulus. Optimal endothelial activation occurred after 2-4 h and 4-6 h using LPS and porin, respectively. Stimulation of leucocytes with either porins or LPS slightly increased their transmigration through non-activated endothelial cells. Transmigration increased remarkably during the simultaneous stimulation of endothelial cells by IL-1ss together with either porins or LPS. To assess participation of E-selectin, intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and leucocyte adhesion complex (CD11/18) in porin- or LPS-mediated leucocyte migration, blocking MoAbs were used. Each blocking MoAb partially and selectively decreased leucocyte transmigration. The obtained results contribute to clarify some aspects of the inflammatory process at sites of infection.
Figures

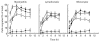



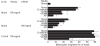
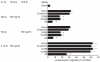

References
-
- Butcher EC. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;20:1033–6. - PubMed
-
- Picker LJ, Warnock RA, Burns AR, Doerschuck CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP140. Cell. 1991;66:921–33. - PubMed
-
- Whisler RL, Cornwell DG, Proctor KV, Downs E. Bacterial lipopolysaccharide acts on human endothelial cells to enhance the adherence of peripheral blood monocytes. J Lab Clin Med. 1989;114:708–16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

