Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model
- PMID: 10362818
- PMCID: PMC1866641
- DOI: 10.1016/S0002-9440(10)65449-1
Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model
Abstract
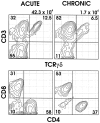
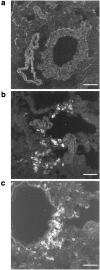
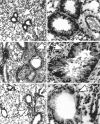
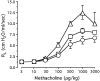
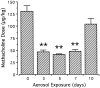
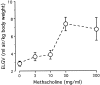
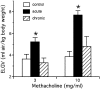
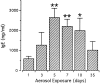
T lymphocytes have a central regulatory role in the pathogenesis of asthma. We delineated the participation of lymphocytes in the acute allergic and chronic tolerant stages of a murine model of asthma by characterizing the various subsets of lymphocytes in bronchoalveolar lavage and lung tissue associated with these responses. Acute (10-day) aerosol challenge of immunized C57BL/6J mice with ovalbumin resulted in airway eosinophilia, histological evidence of peribronchial and perivascular airway inflammation, clusters of B cells and TCRgammadelta cells in lung tissue, increased serum IgE levels, and airway hyperresponsiveness to methacholine. In mice subjected to chronic (6-week) aerosol challenge with ovalbumin, airway inflammation and serum IgE levels were significantly attenuated and airway hyperresponsiveness was absent. The marked increases in lung B and T cell populations seen in the acute stage were also significantly reduced in the chronic stage of this model. Thus, acute ovalbumin challenge resulted in airway sensitization characteristic of asthma, whereas chronic ovalbumin challenge elicited a suppressed or tolerant state. The transition from antigenic sensitization to tolerance was accompanied by shifts in lymphocyte profiles in the lung and bronchoalveolar lavage fluid.
Figures
References
-
- Holt PG: Immunoregulation of the allergic reaction in the respiratory tract. Eur Respir J 1996, 9(Suppl 22):85s-89s - PubMed
-
- Coffman RL, Carty J: A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-γ. J Immunol 1986, 136:949-954 - PubMed
-
- Vercelli D, Geha RS: Regulation of IgE synthesis in humans. J Clin Immunol 1989, 9:75-83 - PubMed
-
- Kay AB: “Helper” (CD4+) T cells and eosinophils in allergy and asthma. Am Rev Respir Dis 1992, 145:S22-S26 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

