Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism
- PMID: 10364159
- PMCID: PMC316780
- DOI: 10.1101/gad.13.11.1422
Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism
Abstract
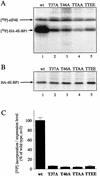
The multisubunit eukaryotic translation initiation factor (eIF) 4F recruits 40S ribosomal subunits to the 5' end of mRNA. The eIF4F subunit eIF4E interacts directly with the mRNA 5' cap structure. Assembly of the eIF4F complex is inhibited by a family of repressor polypeptides, the eIF4E-binding proteins (4E-BPs). Binding of the 4E-BPs to eIF4E is regulated by phosphorylation: Hypophosphorylated 4E-BP isoforms interact strongly with eIF4E, whereas hyperphosphorylated isoforms do not. 4E-BP1 is hypophosphorylated in quiescent cells, but is hyperphosphorylated on multiple sites following exposure to a variety of extracellular stimuli. The PI3-kinase/Akt pathway and the kinase FRAP/mTOR signal to 4E-BP1. FRAP/mTOR has been reported to phosphorylate 4E-BP1 directly in vitro. However, it is not known if FRAP/mTOR is responsible for the phosphorylation of all 4E-BP1 sites, nor which sites must be phosphorylated to release 4E-BP1 from eIF4E. To address these questions, a recombinant FRAP/mTOR protein and a FRAP/mTOR immunoprecipitate were utilized in in vitro kinase assays to phosphorylate 4E-BP1. Phosphopeptide mapping of the in vitro-labeled protein yielded two 4E-BP1 phosphopeptides that comigrated with phosphopeptides produced in vivo. Mass spectrometry analysis indicated that these peptides contain phosphorylated Thr-37 and Thr-46. Thr-37 and Thr-46 are efficiently phosphorylated in vitro by FRAP/mTOR when 4E-BP1 is bound to eIF4E. However, phosphorylation at these sites was not associated with a loss of eIF4E binding. Phosphorylated Thr-37 and Thr-46 are detected in all phosphorylated in vivo 4E-BP1 isoforms, including those that interact with eIF4E. Finally, mutational analysis demonstrated that phosphorylation of Thr-37/Thr-46 is required for subsequent phosphorylation of several carboxy-terminal serum-sensitive sites. Taken together, our results suggest that 4E-BP1 phosphorylation by FRAP/mTOR on Thr-37 and Thr-46 is a priming event for subsequent phosphorylation of the carboxy-terminal serum-sensitive sites.
Figures
















References
-
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. - PubMed
-
- Brooks RF. Continuous protein synthesis is required to maintain the probability of entry into S phase. Cell. 1977;12:311–317. - PubMed
-
- Brown EJ, Beal PA, Keith CT, Chen J, Shin TB, Schreiber SL. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377:441–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
