A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans
- PMID: 10364160
- PMCID: PMC316759
- DOI: 10.1101/gad.13.11.1438
A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans
Abstract
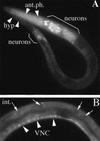
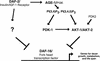
An insulin receptor-like signaling pathway regulates Caenorhabditis elegans metabolism, development, and longevity. Inactivation of the insulin receptor homolog DAF-2, the AGE-1 PI3K, or the AKT-1 and AKT-2 kinases causes a developmental arrest at the dauer stage. A null mutation in the daf-16 Fork head transcription factor alleviates the requirement for signaling through this pathway. We show here that a loss-of-function mutation in pdk-1, the C. elegans homolog of the mammalian Akt/PKB kinase PDK1, results in constitutive arrest at the dauer stage and increased life span; these phenotypes are suppressed by a loss of function mutation in daf-16. An activating mutation in pdk-1 or overexpression of wild-type pdk-1 relieves the requirement for AGE-1 PI3K signaling. Therefore, pdk-1 activity is both necessary and sufficient to propagate AGE-1 PI3K signals in the DAF-2 insulin receptor-like signaling pathway. The activating mutation in pdk-1 requires akt-1 and akt-2 gene activity in order to suppress the dauer arrest phenotype of age-1. This indicates that the major function of C. elegans PDK1 is to transduce signals from AGE-1 to AKT-1 and AKT-2. The activating pdk-1 mutation is located in a conserved region of the kinase domain; the equivalent amino acid substitution in human PDK1 activates its kinase activity toward mammalian Akt/PKB.
Figures



References
-
- Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): Structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997a;7:776–789. - PubMed
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PRJ, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Ba. Curr Biol. 1997b;7:261–269. - PubMed
-
- Alessi DR, Kozlowski MT, Weng QP, Morrice N, Avruch J. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol. 1998;8:69–81. - PubMed
-
- Anderson KE, Coadwell J, Stephens LR, Hawkins PT. Translocation of PDK1 to the plasma membrane is important in allowing PDK1 to activate protein kinase B. Curr Biol. 1998;8:684–691. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
