Targeted expression of SV40 large T-antigen to visceral smooth muscle induces proliferation of contractile smooth muscle cells and results in megacolon
- PMID: 10364214
- PMCID: PMC2824515
- DOI: 10.1074/jbc.274.25.17725
Targeted expression of SV40 large T-antigen to visceral smooth muscle induces proliferation of contractile smooth muscle cells and results in megacolon
Abstract
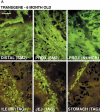
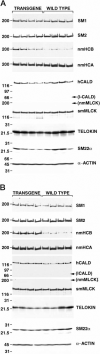
Many pathological conditions result from the proliferation and de-differentiation of smooth muscle cells leading to impaired contractility of the muscle. Here we show that targeted expression of SV40 large T-antigen to visceral smooth muscle cells in vivo results in increased smooth muscle cell proliferation without de-differentiation or decreased contractility. These data suggest that the de-differentiation and proliferation of smooth muscle cells, seen in many pathological states, may be independently regulated. In the T-antigen transgenic mice the increased smooth muscle cell proliferation results in thickening of the distal colon. Consequently the distal colon becomes hyper-contractile and impedes the flow of digesta through the colon resulting in enlargement of the colon oral to the obstruction. These transgenic mice thus represent a novel model of megacolon that results from increased smooth muscle cell proliferation rather than altered neuronal innervation.
Figures





References
-
- Majesky MW, Giachelli CM, Reidy MA, Schwartz SM. Circ. Res. 1992;71:759–768. - PubMed
-
- Herring BP, Smith AF. Am. J. Physiol. 1996;270:C1656–C1665. - PubMed
-
- Aikawa M, Sivan PN, Kuro-o M, Kimura K, Nakahara K-I, Takewaki S-I, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M, Nagai R. Circ. Res. 1993;73:1002–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

