Isolation of recombinant adeno-associated virus vector-cellular DNA junctions from mouse liver
- PMID: 10364291
- PMCID: PMC112600
- DOI: 10.1128/JVI.73.7.5438-5447.1999
Isolation of recombinant adeno-associated virus vector-cellular DNA junctions from mouse liver
Abstract
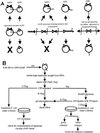
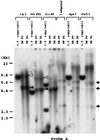
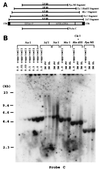
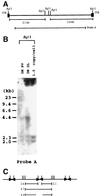
Recombinant adeno-associated virus (rAAV) vectors allow for sustained expression of transgene products from mouse liver following a single portal vein administration. Here a rAAV vector expressing human coagulation factor F.IX (hF.IX), AAV-EF1alpha-F.IX (hF.IX expression was controlled by the human elongation factor 1alpha [EF1alpha] enhancer-promoter) was injected into mice via the portal vein or tail vein, or directly into the liver parenchyma, and the forms of rAAV vector DNA extracted from the liver were analyzed. Southern blot analyses suggested that rAAV vector integrated into the host genome, forming mainly head-to-tail concatemers with occasional deletions of the inverted terminal repeats (ITRs) and their flanking sequences. To further confirm vector integration, we developed a shuttle vector system and isolated and sequenced rAAV vector-cellular DNA junctions from transduced mouse livers. Analysis of 18 junctions revealed various rearrangements, including ITR deletions and amplifications of the vector and cellular DNA sequences. The breakpoints of the vector were mostly located within the ITRs, and cellular DNA sequences were recombined with the vector genome in a nonhomologous manner. Two rAAV-targeted DNA sequences were identified as the mouse rRNA gene and the alpha1 collagen gene. These observations serve as direct evidence of rAAV integration into the host genome of mouse liver and allow us to begin to elucidate the mechanisms involved in rAAV integration into tissues in vivo.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

