Mapping of functional elements in the stem-anchor region of tick-borne encephalitis virus envelope protein E
- PMID: 10364309
- PMCID: PMC112618
- DOI: 10.1128/JVI.73.7.5605-5612.1999
Mapping of functional elements in the stem-anchor region of tick-borne encephalitis virus envelope protein E
Abstract
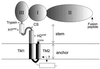
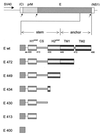
Envelope protein E of the flavivirus tick-borne encephalitis virus mediates membrane fusion, and the structure of the N-terminal 80% of this 496-amino-acid-long protein has been shown to differ significantly from that of other viral fusion proteins. The structure of the carboxy-terminal 20%, the stem-anchor region, is not known. It contains sequences that are important for membrane anchoring, interactions with prM (the precursor of membrane protein M) during virion assembly, and low-pH-induced structural changes associated with the fusion process. To identify specific functional elements in this region, a series of C-terminal deletion mutants were constructed and the properties of the resulting truncated recombinant E proteins were examined. Full-length E proteins and proteins lacking the second of two predicted transmembrane segments were secreted in a particulate form when coexpressed with prM, whereas deletion of both segments resulted in the secretion of soluble homodimeric E proteins. Sites located within a predicted alpha-helical region of the stem (amino acids 431 to 449) and the first membrane-spanning region (amino acids 450 to 472) were found to be important for the stability of the prM-E heterodimer but not essential for prM-mediated intracellular transport and secretion of soluble E proteins. A separate site in the stem, also corresponding to a predicted alpha-helix (amino acids 401 to 413), was essential for the conversion of soluble protein E dimers to a homotrimeric form upon low-pH treatment, a process resembling the transition to the fusogenic state in whole virions. This functional mapping will aid in the understanding of the molecular mechanisms of membrane fusion and virus assembly.
Figures



References
-
- Allison S L, Mandl C W, Kunz C, Heinz F X. Expression of cloned envelope protein genes from the flavivirus tick-borne encephalitis virus in mammalian cells and random mutagenesis by PCR. Virus Genes. 1994;8:187–198. - PubMed
-
- Blacklow S C, Lu M, Kim P S. A trimeric subdomain of the simian immunodeficiency virus envelope glycoprotein. Biochemistry. 1995;34:14955–14962. - PubMed
-
- Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

