Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro
- PMID: 10364346
- PMCID: PMC112655
- DOI: 10.1128/JVI.73.7.5934-5944.1999
Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro
Abstract
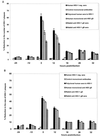
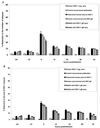
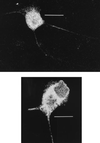
The ability of antibodies to interfere with anterograde transmission of herpes simplex virus (HSV) from neuronal axons to the epidermis was investigated in an in vitro model consisting of human fetal dorsal root ganglia innervating autologous skin explants in a dual-chamber tissue culture system. The number and size of viral cytopathic plaques in epidermal cells after axonal transmission from HSV type 1 (HSV-1)-infected dorsal root ganglionic neurons were significantly reduced by addition to the outer chamber of neutralizing polyclonal human sera to HSV-1, of a human recombinant monoclonal group Ib antibody to glycoprotein D (gD), and of rabbit sera to HSV-1 gB and gD but not by rabbit anti-gE or anti-gG. A similar pattern of inhibition of direct infection of epidermal cells by these antibodies was observed. High concentrations of the monoclonal anti-gD reduced transmission by 90%. Rabbit anti-gB was not taken up into neurons, and human anti-gD did not influence spread of HSV in the dorsal root ganglia or axonal transport of HSV antigens when applied to individual dissociated neurons. These results suggest that anti-gD and -gB antibodies interfere with axonal spread of HSV-1, possibly by neutralizing HSV during transmission across an intercellular gap between axonal termini and epidermal cells, and thus contribute to control of HSV spread and shedding. Therefore, selected human monoclonal antibodies to protective epitopes might even be effective in preventing epidermis-to-neuron transmission during primary HSV infection, especially neonatal infection.
Figures





References
-
- Biron C A, Byron K S, Sullivan J L. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. - PubMed
-
- Brown Z A, Benedetti J, Ashley R, Burchett S, Selke S, Berry S, Vontver L A, Corey L. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247–1252. - PubMed
-
- Burlet A J, Menzaghi F, Tilders F J, Oers J W, Nicholas J P, Burlet C R. Uptake of monoclonal antibody to corticotropin-releasing factor (CRF) into rat hypothalamic neurons. Brain Res. 1990;28:283–293. - PubMed
-
- Corey L, Adams H G, Brown Z A, Holmes K K. Genital herpes simplex virus infection: clinical manifestations, course and complications. Ann Intern Med. 1983;98:958–972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

