Receptor-mediated Moloney murine leukemia virus entry can occur independently of the clathrin-coated-pit-mediated endocytic pathway
- PMID: 10364351
- PMCID: PMC112660
- DOI: 10.1128/JVI.73.7.5994-6005.1999
Receptor-mediated Moloney murine leukemia virus entry can occur independently of the clathrin-coated-pit-mediated endocytic pathway
Abstract
To investigate receptor-mediated Moloney murine leukemia virus (MoMuLV) entry, the green fluorescent protein (GFP)-tagged ecotropic receptor designated murine cationic amino acid transporter (MCAT-1) (MCAT-1-GFP) was constructed and expressed in 293 cells (293/MCAT-1-GFP). 293/MCAT-1-GFP cells displayed green fluorescence primarily at the cell membrane and supported wild-type levels of MoMuLV vector binding and transduction. Using immunofluorescence labeling and confocal microscopy, it was demonstrated that the surface envelope protein (SU) gp70 of MoMuLV virions began to appear inside cells 5 min after virus binding and was colocalized with MCAT-1-GFP. However, clathrin was not colocalized with MCAT-1-GFP, suggesting that MoMuLV entry, mediated by MCAT-1, does not involve clathrin. Double immunofluorescence labeling of SU and clathrin in 293 cells expressing untagged receptor (293/MCAT-1) gave the same results, i.e., SU and clathrin did not colocalize. In addition, we examined the transduction ability of MoMuLV vector on HeLa cells overexpressing the dominant-negative GTPase mutant of dynamin (K44A). HeLa cells overexpressing mutant dynamin have a severe block in endocytosis by the clathrin-coated-pit pathway. No significant titer difference was observed when MoMuLV vector was tranduced into HeLa cells overexpressing either wild-type or mutant dynamin, while the transduction ability of vesicular stomatitis virus glycoprotein pseudotyped vector into HeLa cells overexpressing mutant dynamin was decreased significantly. Taken together, these data suggest that MoMuLV entry does not occur through the clathrin-coated-pit-mediated endocytic pathway.
Figures
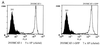
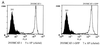






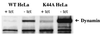
References
-
- Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. - PubMed
-
- Andersen K B, Nexø B A. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983;125:85–98. - PubMed
-
- Berkowitz R D, Goff S P. Point mutations in Moloney murine leukemia virus envelope protein: effects on infectivity, virion association, and superinfection resistance. Virology. 1993;196:748–757. - PubMed
-
- Bruder G, Wiedenmann B. Identification of a distinct 9S form of soluble clathrin in cultured cells and tissues. Exp Cell Res. 1986;164:449–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

