Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion
- PMID: 10364366
- PMCID: PMC112675
- DOI: 10.1128/JVI.73.7.6104-6110.1999
Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion
Abstract
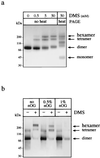
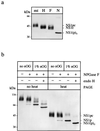
Nonstructural glycoprotein NS1, specified by dengue virus type 1 (Den-1), is secreted from infected green monkey kidney (Vero) cells in a major soluble form characterized by biochemical and biophysical means as a unique hexameric species. This noncovalently bound oligomer is formed by three dimeric subunits and has a molecular mass of 310 kDa and a Stokes radius of 64.4 A. During protein export, one of the two oligosaccharides of NS1 is processed into an endo-beta-N-acetylglucosaminidase F-resistant complex-type sugar while the other remains of the polymannose type, protected in the dimeric subunit from the action of maturation enzymes. Complete processing of the complex-type sugar appears to be required for efficient release of soluble NS1 into the culture fluid of infected cells, as suggested by the repressive effects of the N-glycan processing inhibitors swainsonine and deoxymannojyrimicin. These results, together with observations related to the absence of secretion of NS1 from Den-infected insect cells, suggest that maturation and secretion of hexameric NS1 depend on the glycosylation status of the host cell.
Figures



References
-
- Boulin C, Kempf R, Koch M H J, Mclaughlin S M. Data appraisal, evaluation and display for synchrotron radiation experiments: hardware and software. Nucl Instrum Methods. 1986;A249:399–407.
-
- Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. - PubMed
-
- Crooks A J, Lee J M, Dowsett A B, Stephenson J R. Purification and analysis of infectious virions and native non-structural antigens from cells infected with tick-borne encephalitis virus. J Chromatogr. 1990;502:59–68. - PubMed
-
- Crooks A J, Lee J M, Easterbrook L M, Timofeev A V, Stephenson J R. The NS1 protein of tick-borne encephalitis virus forms multimeric species upon secretion from the host cell. J Gen Virol. 1994;75:3453–3460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

