Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza
- PMID: 10364411
- PMCID: PMC59298
- DOI: 10.1104/pp.120.2.587
Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza
Abstract
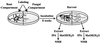
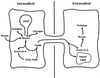
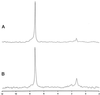
Both the plant and the fungus benefit nutritionally in the arbuscular mycorrhizal symbiosis: The host plant enjoys enhanced mineral uptake and the fungus receives fixed carbon. In this exchange the uptake, metabolism, and translocation of carbon by the fungal partner are poorly understood. We therefore analyzed the fate of isotopically labeled substrates in an arbuscular mycorrhiza (in vitro cultures of Ri T-DNA-transformed carrot [Daucus carota] roots colonized by Glomus intraradices) using nuclear magnetic resonance spectroscopy. Labeling patterns observed in lipids and carbohydrates after substrates were supplied to the mycorrhizal roots or the extraradical mycelium indicated that: (a) 13C-labeled glucose and fructose (but not mannitol or succinate) are effectively taken up by the fungus within the root and are metabolized to yield labeled carbohydrates and lipids; (b) the extraradical mycelium does not use exogenous sugars for catabolism, storage, or transfer to the host; (c) the fungus converts sugars taken up in the root compartment into lipids that are then translocated to the extraradical mycelium (there being little or no lipid synthesis in the external mycelium); and (d) hexose in fungal tissue undergoes substantially higher fluxes through an oxidative pentose phosphate pathway than does hexose in the host plant.
Figures


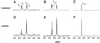

Similar articles
-
The fungus does not transfer carbon to or between roots in an arbuscular mycorrhizal symbiosis.New Phytol. 2004 Sep;163(3):617-627. doi: 10.1111/j.1469-8137.2004.01152.x. New Phytol. 2004. PMID: 33873744
-
Nitrogen transfer and assimilation between the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith and Ri T-DNA roots of Daucus carota L. in an in vitro compartmented system.Can J Microbiol. 2004 Apr;50(4):251-60. doi: 10.1139/w04-009. Can J Microbiol. 2004. PMID: 15213749
-
Lead uptake by the symbiotic Daucus carota L.-Glomus intraradices system and its effect on the morphology of extra- and intraradical fungal microstructures.Environ Sci Pollut Res Int. 2019 Jan;26(1):381-391. doi: 10.1007/s11356-018-3569-7. Epub 2018 Nov 6. Environ Sci Pollut Res Int. 2019. PMID: 30402695
-
Forms of nitrogen uptake, translocation, and transfer via arbuscular mycorrhizal fungi: a review.Sci China Life Sci. 2012 Jun;55(6):474-82. doi: 10.1007/s11427-012-4330-y. Epub 2012 Jun 29. Sci China Life Sci. 2012. PMID: 22744177 Review.
-
Diet of Arbuscular Mycorrhizal Fungi: Bread and Butter?Trends Plant Sci. 2017 Aug;22(8):652-660. doi: 10.1016/j.tplants.2017.05.008. Epub 2017 Jun 13. Trends Plant Sci. 2017. PMID: 28622919 Review.
Cited by
-
Fungal root symbionts of high-altitude vascular plants in the Himalayas.Sci Rep. 2017 Jul 26;7(1):6562. doi: 10.1038/s41598-017-06938-x. Sci Rep. 2017. PMID: 28747779 Free PMC article.
-
Arbuscular mycorrhizal fungi favor invasive Echinops sphaerocephalus when grown in competition with native Inula conyzae.Sci Rep. 2020 Nov 20;10(1):20287. doi: 10.1038/s41598-020-77030-0. Sci Rep. 2020. PMID: 33219310 Free PMC article.
-
Contrasting responses to mycorrhizal inoculation and phosphorus availability in seedlings of two tropical rainforest tree species.New Phytol. 2004 Mar;161(3):865-875. doi: 10.1046/j.1469-8137.2004.00978.x. Epub 2004 Jan 8. New Phytol. 2004. PMID: 33873722
-
In Vitro Mycorrhization for Plant Propagation and Enhanced Resilience to Environmental Stress: A Review.Plants (Basel). 2025 Jul 8;14(14):2097. doi: 10.3390/plants14142097. Plants (Basel). 2025. PMID: 40733334 Free PMC article. Review.
-
The fungus does not transfer carbon to or between roots in an arbuscular mycorrhizal symbiosis.New Phytol. 2004 Sep;163(3):617-627. doi: 10.1111/j.1469-8137.2004.01152.x. New Phytol. 2004. PMID: 33873744
References
-
- Amijee F, Stribley DP. Soluble carbohydrates of vesicular-arbuscular mycorrhizal fungi. Mycologist. 1987;21:21–22.
-
- Bécard G, Doner LW, Rolin DB, Douds DD, Pfeffer PE. Identification and quantification of trehalose in vesicular-arbuscular mycorrhizal fungi by in vivo 13C NMR and HPLC analyses. New Phytol. 1991;118:547–552.
-
- Bécard G, Fortin JA. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 1988;108:211–218. - PubMed
-
- Bécard G, Piché Y. Establishment of vesicular-arbuscular mycorrhiza in root organ culture: review and proposed methodology. Methods Microbiol. 1992;24:89–108.
-
- Bonfonte P, Balestrini R, Mendgen K. Storage and secretion processes in the spore of Gigaspora margarita Becker and Hall as revealed by high-pressure freezing and freeze substitution. New Phytol. 1994;128:93–101. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

