Detection of equine antibodies to babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in a competitive-inhibition enzyme-linked immunosorbent assay
- PMID: 10364599
- PMCID: PMC85139
- DOI: 10.1128/JCM.37.7.2285-2290.1999
Detection of equine antibodies to babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in a competitive-inhibition enzyme-linked immunosorbent assay
Abstract
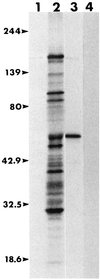
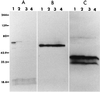
A competitive-inhibition enzyme-linked immunosorbent assay (cELISA) was developed for detection of equine antibodies specific for Babesia caballi. The assay used recombinant B. caballi rhoptry-associated protein 1 (RAP-1) and monoclonal antibody (MAb) 79/17.18.5, which is reactive with a peptide epitope of a native 60-kDa B. caballi antigen. The gene encoding the recombinant antigen was sequenced, and database analysis revealed that the gene product is a rhoptry-associated protein. Cloning and expression of a truncated copy of the gene demonstrated that MAb 79/17.18.5 reacts with the C-terminal repeat region of the protein. The cELISA was used to evaluate 302 equine serum samples previously tested for antibodies to B. caballi by a standardized complement fixation test (CFT). The results of cELISA and CFT were 73% concordant. Seventy-two of the 77 serum samples with discordant results were CFT negative and cELISA positive. Further evaluation of the serum samples with discordant results by indirect immunofluorescence assay (IFA) demonstrated that at a serum dilution of 1:200, 48 of the CFT-negative and cELISA-positive serum samples contained antibodies reactive with B. caballi RAP-1. Four of five CFT-positive and cELISA-negative serum samples contained antibodies reactive with B. caballi when they were tested by IFA. These data indicate that following infection with B. caballi, horses consistently produce antibody to the RAP-1 epitope defined by MAb 79/17.18.5, and when used in the cELISA format, recombinant RAP-1 is a useful antigen for the serologic detection of anti-B. caballi antibodies.
Figures
Similar articles
-
Serum antibodies from a subset of horses positive for Babesia caballi by competitive enzyme-linked immunosorbent assay demonstrate a protein recognition pattern that is not consistent with infection.Clin Vaccine Immunol. 2013 Nov;20(11):1752-7. doi: 10.1128/CVI.00479-13. Epub 2013 Sep 18. Clin Vaccine Immunol. 2013. PMID: 24049108 Free PMC article.
-
Sequence heterogeneity in the gene encoding the rhoptry-associated protein-1 (RAP-1) of Babesia caballi isolates from South Africa.Vet Parasitol. 2010 May 11;169(3-4):279-88. doi: 10.1016/j.vetpar.2010.01.009. Epub 2010 Jan 20. Vet Parasitol. 2010. PMID: 20138703
-
Cloning and expression of a 48-kilodalton Babesia caballi merozoite rhoptry protein and potential use of the recombinant antigen in an enzyme-linked immunosorbent assay.J Clin Microbiol. 1999 Nov;37(11):3475-80. doi: 10.1128/JCM.37.11.3475-3480.1999. J Clin Microbiol. 1999. PMID: 10523537 Free PMC article.
-
Molecular cloning of a Babesia caballi gene encoding the 134-kilodalton protein and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay.Clin Diagn Lab Immunol. 2004 Jan;11(1):211-5. doi: 10.1128/cdli.11.1.211-215.2004. Clin Diagn Lab Immunol. 2004. PMID: 14715570 Free PMC article.
-
Immunochromatographic test for simultaneous serodiagnosis of Babesia caballi and B. equi infections in horses.Clin Vaccine Immunol. 2006 May;13(5):553-5. doi: 10.1128/CVI.13.5.553-555.2006. Clin Vaccine Immunol. 2006. PMID: 16682475 Free PMC article.
Cited by
-
Improved enzyme-linked immunosorbent assay using C-terminal truncated recombinant antigens of Babesia bovis rhoptry-associated protein-1 for detection of specific antibodies.J Clin Microbiol. 2004 Apr;42(4):1601-4. doi: 10.1128/JCM.42.4.1601-1604.2004. J Clin Microbiol. 2004. PMID: 15071011 Free PMC article.
-
Epidemiological Study of Equine Piroplasmosis (Theileria equi and Babesia caballi) by Microscopic Examination and Competitive-ELISA in Erbil Province North-Iraq.Iran J Parasitol. 2019 Jul-Sep;14(3):404-412. Iran J Parasitol. 2019. PMID: 31673258 Free PMC article.
-
Competitive enzyme-linked immunosorbent assay based on a rhoptry-associated protein 1 epitope specifically identifies Babesia bovis-infected cattle.Clin Diagn Lab Immunol. 2003 Jan;10(1):38-43. doi: 10.1128/cdli.10.1.38-43.2003. Clin Diagn Lab Immunol. 2003. PMID: 12522037 Free PMC article.
-
Research progress on diagnostic techniques for different Babesia species in persistent infections.Front Cell Infect Microbiol. 2025 May 16;15:1575227. doi: 10.3389/fcimb.2025.1575227. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40453708 Free PMC article. Review.
-
The invasion process of bovine erythrocyte by Babesia divergens: knowledge from an in vitro assay.Vet Res. 2011 May 11;42(1):62. doi: 10.1186/1297-9716-42-62. Vet Res. 2011. PMID: 21569363 Free PMC article.
References
-
- Altschul S F, Gish W, Miller W, Meyers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Bose R, Daeman K. Demonstration of the humoral immune response of horses to Babesia caballi by Western blotting. Int J Parasitol. 1992;22:627–630. - PubMed
-
- Bose R. Polyclonal antibody characterization of Babesia caballi antigens. Int J Parasitol. 1994;24:511–517. - PubMed
-
- Frerichs W M, Holbrook A A, Johnson A J. Equine piroplasmosis: complement fixation titers of horses infected with Babesia caballi. Am J Vet Res. 1969;30:697–702. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

