Presynaptic mu and delta opioid receptor modulation of GABAA IPSCs in the rat globus pallidus in vitro
- PMID: 10366614
- PMCID: PMC6782644
- DOI: 10.1523/JNEUROSCI.19-12-04796.1999
Presynaptic mu and delta opioid receptor modulation of GABAA IPSCs in the rat globus pallidus in vitro
Abstract
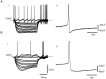
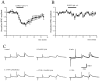
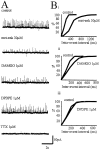
The role of enkephalin and the opioid receptors in modulating GABA release within the rat globus pallidus (GP) was investigated using whole-cell patch recordings made from visually identified neurons. Two major GP neuronal subtypes were classified on the basis of intrinsic membrane properties, action potential characteristics, the presence of the anomalous inward rectifier (Ih), and anode break depolarizations. The mu opioid receptor agonist [D-Ala2-N-Me-Phe4-Glycol5]-enkephalin (DAMGO) (1 microM) reduced GABAA receptor-mediated IPSCs evoked by stimulation within the striatum. DAMGO also increased paired-pulse facilitation, indicative of presynaptic mu opioid receptor modulation of striatopallidal input. In contrast, the delta opioid agonist D-Pen-[D-Pen2, 5]-enkephalin (DPDPE) (1 microM) was without effect. IPSCs evoked by stimulation within the GP were depressed by application of [methionine 5']-enkephalin (met-enkephalin) (30 microM). Met-enkephalin also reduced the frequency, but not the amplitude, of miniature IPSCs (mIPSCs) and increased paired-pulse facilitation of evoked IPSCs, indicative of a presynaptic action. Both DAMGO and DPDPE reduced evoked IPSCs and the frequency, but not amplitude, of mIPSCs. However, spontaneous action potential-driven IPSCs were reduced in frequency by met-enkephalin and DAMGO, whereas DPDPE was without effect. Overall, these results indicate that presynaptic mu opioid receptors are located on striatopallidal terminals and pallidopallidal terminals of spontaneously firing GP neurons, whereas presynaptic delta opioid receptors are preferentially located on terminals of quiescent GP cells. Enkephalin, acting at both of these receptor subtypes, serves to reduce GABA release in the GP and may therefore act as an adaptive mechanism, maintaining the inhibitory function of the GP in basal ganglia circuitry.
Figures



Similar articles
-
Glycine and GABAA receptor-mediated synaptic transmission in rat substantia gelatinosa: inhibition by mu-opioid and GABAB agonists.J Physiol. 1998 Mar 1;507 ( Pt 2)(Pt 2):473-83. doi: 10.1111/j.1469-7793.1998.473bt.x. J Physiol. 1998. PMID: 9518706 Free PMC article.
-
Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro.Br J Pharmacol. 1994 Sep;113(1):303-9. doi: 10.1111/j.1476-5381.1994.tb16209.x. Br J Pharmacol. 1994. PMID: 7812626 Free PMC article.
-
Activation of mu-opioid receptors excites a population of locus coeruleus-spinal neurons through presynaptic disinhibition.Brain Res. 2004 Jan 30;997(1):67-78. doi: 10.1016/j.brainres.2003.10.050. Brain Res. 2004. PMID: 14715151
-
Key differences in regulation of opioid receptors localized to presynaptic terminals compared to somas: Relevance for novel therapeutics.Neuropharmacology. 2023 Mar 15;226:109408. doi: 10.1016/j.neuropharm.2022.109408. Epub 2022 Dec 28. Neuropharmacology. 2023. PMID: 36584882 Free PMC article. Review.
-
The globus pallidus as a target for neuropeptides and endocannabinoids participating in central activities.Peptides. 2020 Feb;124:170210. doi: 10.1016/j.peptides.2019.170210. Epub 2019 Nov 26. Peptides. 2020. PMID: 31778724 Review.
Cited by
-
Firing rate and pattern heterogeneity in the globus pallidus arise from a single neuronal population.J Neurophysiol. 2013 Jan;109(2):497-506. doi: 10.1152/jn.00677.2012. Epub 2012 Oct 31. J Neurophysiol. 2013. PMID: 23114208 Free PMC article.
-
Autoradiographic analysis of GABAA receptors in mu-opioid receptor knockout mice.Neurochem Res. 2007 Nov;32(11):1891-7. doi: 10.1007/s11064-007-9373-2. Epub 2007 Jun 12. Neurochem Res. 2007. PMID: 17562169
-
Adenosine A(2A) receptor enhances GABA(A)-mediated IPSCs in the rat globus pallidus.J Physiol. 2001 Apr 15;532(Pt 2):423-34. doi: 10.1111/j.1469-7793.2001.0423f.x. J Physiol. 2001. PMID: 11306661 Free PMC article.
-
Roles of micro-opioid receptors in GABAergic synaptic transmission in the striosome and matrix compartments of the striatum.Mol Neurobiol. 2008 Apr-Jun;37(2-3):104-15. doi: 10.1007/s12035-008-8023-2. Epub 2008 May 13. Mol Neurobiol. 2008. PMID: 18473190 Review.
-
The effects of DBS patterns on basal ganglia activity and thalamic relay : a computational study.J Comput Neurosci. 2012 Aug;33(1):151-67. doi: 10.1007/s10827-011-0379-z. Epub 2012 Jan 13. J Comput Neurosci. 2012. PMID: 22237601
References
-
- Abou Khalil B, Young AB, Penney JB. Evidence for the presynaptic localization of opiate binding sites on striatal efferent fibers. Brain Res. 1984;323:21–29. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Asselin MC, Soghomonian JJ, Cote P, Parent A. Striatal changes in preproenkephalin mRNA levels in parkinsonian monkeys. NeuroReport. 1994;5:2137–2140. - PubMed
-
- Augood SJ, Emson P, Mitchell IJ, Boyce S, Clarke CE, Crossman AR. Cellular localisation of enkephalin gene expression in MPTP-treated cynomolgus monkeys. Mol Brain Res. 1989;6:85–92. - PubMed
-
- Austin MC, Kalivas PW. Enkephalinergic and GABAergic modulation of motor activity in the ventral pallidum. J Pharm Exp Ther. 1990;252:1370–1377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
